Carbohydrates are an important type of biomacromolecules in nature. The basic building blocks of carbohydrates are monosaccharides, which commonly exist as pyranose ring structure in nature. It is well known that the common monosaccharides are stereoisomers, for example, glucose, galactose and mannose are stereoisomers with only difference coming from the orientation of hydroxyl groups. But these differences change the intermolecular hydrogen bonds between carbohydrates, resulting different carbohydrate-carbohydrate interactions (CCI). In our previous studies, we noticed this phenomenon and constructed various glycopolymers to demonstrate the different properties oriented from carbohydrates [1-5]. For example, we found that with crystalline polymeric backbones, mannoside modification helped the polymer to maintain its crystalline nature, while galactoside modification made the crystalline polymer become amorphous [5]. Besides, glucoside or galactoside modifications would also equip polymers with various assembling morphologies and special biological applications [3, 6]. To further decipher the role of monosaccharides, in this work we tend to employ small molecular systems to study the effect of various monosaccharides on selfassembly.
For the investigation of monosaccharide on self-assembly, we chose a small molecule, 1, 3, 5-benzenetricarboamides (BTAs), to perform the research on monosaccharide effect on self-assembly. It should be noticed that some common monosaccharide-containing small molecules (such as glycolipid [7, 8] and monosaccharidecontaining perylene bisimide derivative [9-11]) have been employed to study the effect of monosaccharide, but these compounds showed relatively low carbohydrate content and monotonous assembly morphology. Thus, we pursued a small molecular system with relatively high carbohydrate content, probable abundant assembly morphology and length-adjustable linkers. BTAs are regarded as one of the most popular supramolecular monomers which can assemble into abundant nanostructures [12-15]. With hydrophobic interactions and hydrogen bonds of this small molecule, BTAs favor to assemble into nanofibers, as a stable and common morphology of assemblies.
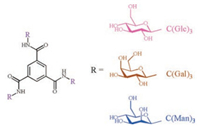
Meanwhile, the morphologies could be regulated by inducing different side chains, forming various helical nanofibers. The stability and variety of BTA assemblies indicated this small molecular structure was appropriate to investigate the effect of monosaccharides. What is more, one BTA molecule could link three monosaccharides, the high monosaccharide content is beneficial to amplify the monosaccharide effect on assemblies. In 2016, Meijer et al. investigated the synthesis and the assembly of monosaccharide-containing BTAs which had a long alkyl chain between carbohydrate and BTA cores [16]. With different monosaccharides, all the BTAs assembled into similar nanofibers, indicating that monosaccharide made little contribution to the assembly morphology. Thus in this paper, we design a new monosaccharidecontaining BTA, in which carbohydrates are linked to the core of BTA by amide bonds. Three monosaccharides (glucose/Glc, galactose/Gal and mannose/Man) were connected to the same BTA core, forming three different molecules (Fig. 1), C(Glc)3, C(Gal)3 and C(Man)3, respectively. Every monosaccharide BTA had one kind of carbohydrate side chains, these structures could demonstrate the effect of monosaccharides on assembly morphology. With three different monosaccharide side groups, we attempted to study the effect of monosaccharides on the morphology and the process of assembly of monosaccharidecontaining BTAs in aqueous solution. Furthermore, between carbohydrate and BTA cores, segments of alanine residues were introduced to weaken the effect of monosaccharides, which might resulting in shape change compared to monosaccharide-containing BTAs without alanine linkers.
|
Download:
|
| Fig. 1. Chemical structures of carbohydrate 1, 3, 5-benzenetricarboxamides. C(Glc)3 (in pink), C(Gal)3 (in brown) and C(Man)3 (in blue). | |
Before the investigation of assembly, we should design a feasible route of synthesis. Our synthetic strategy was completing the preparation of monosaccharide-containing side chains firstly, then sides chains were connected to the BTA core (Scheme 1). For monosaccharide-based BTAs without alanine linkers, syntheses of C(Glc)3 and C(Gal)3 were following a same route (Scheme 1).
|
Download:
|
| Scheme 1. Chemical structures and a brief synthetic route (take glucose for example) of nine monosaccharide-based BTAs. | |
Taking glucose (1) as an example, the compound 1 underwent an acetylation (2a) firstly. After bromination (2b), substitution with azide (2c) and reduction (2d) successively, glucose-containing side chains were linked to BTA cores via amidation (3a). The final product was obtained (3b, β-configuration) after deprotection. C(Man)3 (β-configurations) was prepared via acetylation, bromination, substitution with azide, deprotection, reduction and amidation in sequence. In the synthesis of C(Man)3, the product of substitution with azide contained a pair of diastereoisomers (α: β = 1:1), the β-configuration was obtained by silica column chromatography. For the carbohydrate-based BTA with alanine linkers, after reduction (5b), the glucoside derivative was connected to alanine (6a) or alanine dipeptide (6b). After the deprotection of the amino group (6b) and the amidation with the BTA core, the final compound was obtained (7). All of the carbohydrate-BTAs with alanine linkers could be prepared via this route. The synthesis details and characterization results were shown in Supporting Material.
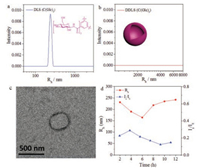
With the compounds in hand, the effect of monosaccharides on assembly was first demonstrated by using molecules C(Glc)3, C(Gal)3 and C(Man)3, without alanine linkers between the monosaccharides and the BTA cores. All these assembly experiments were performed under the same condition with same concentration to compare the effect from monosaccharides. It was found that C(Glc)3 self-assembled into monodispersed nanoobjects in aqueous solution after 12 h, with the hydrodynamic radius (<Rh>) about 242 nm (Fig. 2a). TEM indicated that the morphology was vesicle with diameter in accordance to the DLS results. Moreover, there was no signal in the data of DDLS (Fig. 2b), which stated that the assembly of C(Glc)3 was isotropy, consistent to the vesicular morphology provided by DLS and TEM (Fig. 2c). From the variation of <Rh> values in 12 h (Fig. 2d), C(Glc)3 was found to aggregate into assemblies with a <Rh> value at about 240 nm rapidly, the value showed little difference in the process from 2 h to 12 h, as well as the scattered light intensity (Is/I0). We speculated, under the influence of the effect of glucose, C(Glc)3 could self-assemble into spherical vesicles with a diameter close to 500 nm.
|
Download:
|
| Fig. 2. Assembly of C(Glc)3 in 12 h. DLS (a) and DDLS (b) data after 12 h assembly. TEM image (c) after 12 h. DLS tracing data (d) from 2 h to 12 h. | |
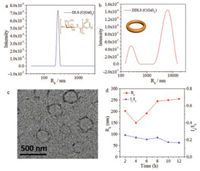
Slightly distinguished from C(Glc)3, C(Gal)3 self-assembled into nano-objects in aqueous solution after 12 h, the <Rh> value was about 255 nm (Fig. 3a). TEM stated this nano-object was nanoring and the diameter was about 500 nm (Fig. 3c). In the DDLS results (Fig. 3b), two peaks were observed, which indicated that the assembly was anisotropic, instead of an isotropic spheres, supporting the nanoring morphologies. From the time-dependent DLS experiment (Fig. 3d), both the <Rh> and Is/I0 values showed little variation in 12 h, similar to the results of C(Glc)3. We supposed that, based on the effect of galactoside, C(Gal)3 assembled into nanorings, distinct from the morphology of C(Glc)3 at the same condition.
|
Download:
|
| Fig. 3. Assembly of C(Gal)3 in 12 h. DLS (a) and DDLS (b) data after 12 h assembly. TEM image (c) after 12 h. DLS tracing data (d) from 2 h to 12 h. | |
Surprisingly, the assembly of C(Man)3 showed significant difference from both C(Glc)3 and C(Gal)3. After 12 h, C(Man)3 assembled into nanostructures with a very large <Rh> value (Fig. 4a), meanwhile TEM images exhibited long twisted nanofibers (Fig. 4c). The morphology of C(Man)3 at 12 h was anisotropic, resulting in the appearance of a peak in DDLS data (Fig. 4b). From the evolution experiment in 12 h (Fig. 4d), <Rh> and Is/I0 values showed an increase from 2 h to 12 h, which stated C(Man)3 had a more obvious tendency to self-assemble into micrometer-scale structures compared with C(Glc)3 and C(Gal)3. This distinct tendency might arise from the special effect of mannoside. From the experiment we suspected, the effect of mannoside was beneficial to the assembly of monosaccharide BTA molecules. The special effect of mannoside might result from the unique configuration of mannoside, i.e., most hydroxyl groups toward the same side of the six-membered ring, resulting a relatively hydrophobic face on one side of the ring, which promoted hydrophobic interactions. This configuration could enhance the assembly of monosaccharide BTAs in 1D direction, thus C(Man)3 assembled into long twisted fibers in micron scale. For glucose (Fig. S6a in Supporting information), hydroxide groups were distributed on both sides of six-membered rings equably, thus the tendency of monosaccharide BTA assemblies was weak and the assembly of C(Glc)3 was vesicle in nanometer scale. For galactose (Fig. S6b), though most hydroxyl groups oriented to one side of sixmembered rings, the hydrophobic plane located quite far from BTA core, while the hydrophobic plane of mannose close to the BTA core (Fig. S6c). Thus the structure of C(Gal)3 was not beneficial to the 1D direction assembly as the structure of C(Man)3 was. Although the results seemed interesting and may not be explained well at the current stage, it was obvious that the difference of assembly morphologies resulted from the various effects of monosaccharides, i.e., the configuration of monosaccharide played a key role in monosaccharide BTA assembly.
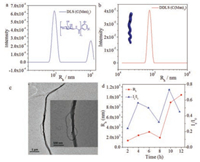
|
Download:
|
| Fig. 4. Assembly of C(Man)3 in 12 h. DLS (a) and DDLS (b) data after 12 h assembly. TEM image (c) after 12 h. DLS tracing data (d) from 2 h to 12 h. | |
To further demonstrate the effect of carbohydrates, a control molecule C(TEG)3 (the chemical structure was shown in Fig. 5a), with ethylene oxide chain replacing carbohydrates, was synthesized and assembled by a similar experimental process. Following the same condition, the assembly of C(TEG)3 was prepared in aqueous solution for 12 h. In contrast, C(TEG)3 assembled into polydispersed spherical nanoparticles with diameter about 486 nm (Figs. 5a-c). The morphology was quite different from vesicles, nanorings and twisted fibers. It demonstrated that the effect of monosaccharide indeed could promote BTAs to form regular assembly morphologies, further supported the effect of monosaccharides.
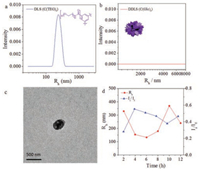
|
Download:
|
| Fig. 5. Assembly of C(TEG)3 in 12 h. DLS (a) and DDLS (b) data after 12 h assembly. TEM image (c) after 12 h. DLS tracing data (d) from 2 h to 12 h. | |
To the further investigate the effect of monosaccharides on the assembly of BTA-containing molecules, an alanine-containing linker (alanine, A) between monosaccharides and benzene cores in BTAs was employed. By this way, we tend to increase the interactions between peptide segments, relatively weaken the interactions between monosaccharides to further demonstrate the effect of monosaccharides.
With one alanine residue between monosaccharides and BTA cores, C(AGlc)3 self-assembled into irregular particles after 12 h, the average diameter of aggregates was 500 nm (Fig. 6a, Fig. S2a). During the process of assembly, <Rh> values did not change significantly, which was similar to the assembly of C(Glc)3 (Fig. 6a). When the linker had two alanine residues, C(A2Glc)3 formed some irregular aggregates with a diameter at about 400 nm (Fig. 6a, Fig. S2b) after 12 h. In the assembling process, <Rh> value showed a slight increase, this might result from the growth of the assembly via alanine likers. Compared with C(Glc)3 with the peptide segments, C(AGlc)3 and C(A2Glc)3 formed some irregular aggregates. This variation indicated, with the enhancement of the interaction between BTA cores, morphology of the glucosebased BTA transformed from vesicles to disordered structures.

|
Download:
|
| Fig. 6. Assemblies of monosaccharide BTAs with alanine linkers in 12 h. DLS tracing data of glucose-based (a), galactose-based (b) and mannose-based (c) BTAs from 2 h to 12 h. | |
After 12 h in aqueous solution, C(AGal)3 formed into irregular fibrous structures, the diameters of these structures showed a board distribution from TEM images (Fig. 6b, Fig. S2c). The <Rh> value exhibited a moderate growth from 0 h to 12 h, when one alanine residue was introduced as a linker. To C(A2Gal)3, it aggregated into irregular particles with a diameter about 800 nm after 12 h in aqueous solution (Fig. 6b, Fig. S2d). For galactosidebased BTAs, with the increase of the number of alanine, they aggregated into some irregular morphologies, such as fibrous structures and irregular particles. By the enhancement of BTA core interaction, galactose-based BTAs formed into a micron scale structure with high polydispersity. In the case of C(AMan)3, they aggregated into irregular nanofibers in aqueous solution after 12 h (Fig. 6c, Fig. S2e). The <Rh> value of the irregular fiber was 1000 nm approximately (Fig. 6g). In addition, C(A2Man)3 formed into similar irregular aggregates (Fig. 6c, Fig. S2f), compared to C(A2Glc)3 and C(A2Gal)3. To the assembly of mannose-based BTAs, the strengthen of core interactions was not beneficial to long fibrous morphology fromation C(AMan)3 in micron scale resulting irregular clusters in contrast. Along with the enhancement of core interactions sequentially, C(A2Man)3 formed into irregular aggregates. It was worth noting that all the three monosaccharide BTAs aggregated into disordered structures, we suppose that this phenomenon was mainly controlled by BTA core interactions instead of carbohydrates.
Among all of the monosaccharide-based BTA molecules, only C(Man)3 formed twisted fibers with a micrometer length. When monosaccharides were linked to BTA cores directly, C(Man)3 selfassembled into fibers in micron scale while C(Glc)3 and C(Gal)3 formed into vesicles and nanorings respectively. The <Rh> value also showed an obvious variation among three monosaccharidemodified BTAs (Fig. 7a) as well as UV-vis spectrum (Fig. 7b) after 12 h. C(Man)3 exhibited an obvious broad peak from other monosaccharide-based BTAs for its good assembly ability. It was indicated that, compared to C(Glc)3 and C(Gal)3, only the interaction between mannoside could benefit the formation of assemblies in micro scale. Furthermore, when alanine side chains were introduced, all three types of monosaccharide-based BTAs aggregated into some irregular morphologies gradually. Similar disordered structures from different monosaccharide-based molecules were observed when the number of alanine residues was increased to two. We concluded that the existence of alanine side chains could decrease the effect of the interaction between monosaccharides, so the morphologies of aggregates turned out to be similar.

|
Download:
|
| Fig. 7. <Rh> values of different monosaccharide BTAs after 12 h (a). UV-vis spectrum of different monosaccharide BTAs after 12 h (b). | |
In summary, based on the different effect of monosaccharides, three monosaccharide BTAs (C(Glc)3, C(Gal)3 and C(Man)3) aggregated into vesicles, nanorings and twisted fibers, respectively. Among these molecules only C(Man)3 formed into micron-scale structures while the other assemblies were in nanoscale. It was indicated that the effect of mannoside was beneficial to the assembly of monosaccharide-based BTAs compared to glucoside and galactoside. With the presence and the growth of alanine linkers, all the assembly morphologies became disordered, when the number of alanine residues was changed to two, the three monosaccharide-based BTAs formed into similar irregular aggregates. These results indicated the previous overlooked effects from different monosaccharides in assembly in literature, which should be useful in the design of new carbohydrate-based materials.
AcknowledgmentsThe Ministry of Science and Technology of China and the National Natural Science Foundation of China (Nos. 91527305 and 51322306) are acknowledged for their financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.12.014.
| [1] |
L. Su, Y. Zhao, G. Chen, M. Jiang, Polym. Chem. 3 (2012) 1560-1566. DOI:10.1039/c2py20110k |
| [2] |
L. Su, W. Zhang, X. Wu, et al., Small 11 (2015) 4191-4200. DOI:10.1002/smll.v11.33 |
| [3] |
P. Sun, M. Lin, Y. Zhao, G. Chen, M. Jiang, Colloid Surf. B 133 (2015) 12-18. DOI:10.1016/j.colsurfb.2015.05.036 |
| [4] |
P. Sun, M. Lin, G. Chen, M. Jiang, Sci. China Chem. 59 (2016) 1616-1620. DOI:10.1007/s11426-016-0117-5 |
| [5] |
L. Wu, Y. Zhang, Z. Li, et al., J. Am. Chem. Soc. 139 (2017) 14684-14692. DOI:10.1021/jacs.7b07768 |
| [6] |
M. Lin, Y. Zhang, G. Chen, M. Jiang, Small 11 (2015) 6065-6070. DOI:10.1002/smll.201501871 |
| [7] |
L.J. Johnston, Langmuir 23 (2007) 5886-5895. DOI:10.1021/la070108t |
| [8] |
M.L. DeMarco, Biochemistry 51 (2012) 5725-5732. DOI:10.1021/bi3003633 |
| [9] |
K.R. Wang, D. Han, G.J. Cao, X.L. Li, RSC Adv. 5 (2015) 47728-47731. DOI:10.1039/C5RA06255A |
| [10] |
K.R. Wang, H.W. An, Y.Q. Wang, J.C. Zhang, X.L. Li, Org. Biomol. Chem. 11 (2013) 1007-1012. DOI:10.1039/c2ob27052h |
| [11] |
K.R. Wang, H.W. An, F. Qian, et al., RSC Adv. 3 (2013) 23190-23196. DOI:10.1039/c3ra44675a |
| [12] |
M.M. Smulders, A.P. Schenning, E.W. Meijer, J. Am. Chem. Soc. 130 (2008) 606-611. DOI:10.1021/ja075987k |
| [13] |
S. Cantekin, Y. Nakano, J.C. Everts, et al., Chem. Commun. 48 (2012) 3803-3805. DOI:10.1039/c2cc17284d |
| [14] |
M.B. Baker, L. Albertazzi, I.K. Voets, et al., Nat. Commun. 6 (2015) 7234-7245. DOI:10.1038/ncomms8234 |
| [15] |
M. Garzoni, M.B. Baker, C.M.A. Leenders, et al., J. Am. Chem. Soc. 138 (2016) 13985-13995. DOI:10.1021/jacs.6b07530 |
| [16] |
C.M.A. Leenders, G. Jansen, M.M.M. Frissen, et al., Chem.-Eur. J. 22 (2016) 4608-4615. DOI:10.1002/chem.201504762 |
 2019, Vol. 30
2019, Vol. 30 

