b Institute of Environmental Sciences, Department of Chemistry, Shanxi University, Taiyuan 030006, China;
c Comprehensive Analysis Center, ISIR, Osaka University, Mihogaoka, Ibaraki 567-0047, Japan;
d Center for Supramolecular Chemistry, International Research Center and Department of Chemistry, Kalasalingam University, Krishnankoil, Tamil Nadu 626126, India
Precisely operating molecular mechanical devices is an intriguing topic of current nanotechnology. The synthesis of molecular motors has attracted considerable attention and a variety of molecular machines on the basis of interlocked molecules, such as rotaxanes, catenanes and molecular knots, have been designed and constructed [1]. Several strategies, including electrochemical and photochemical control, have been applied to drive the motion of molecular motors [2]. Artificial supramolecular chemical structures based on rotaxanes belong to the most extensively studied molecular machines, because of their interesting mechanical interlocking structures, unique physical/chemical properties and a wide range of potential applications. The non-covalent interlocked feature makes the ring freely slidable along the axle, and most studies on the rotaxane-based molecular machines focused on the function arising from the slide motion of the ring along the axle. On the other hand, the study on controlling the rotational kinetic of the ring around the axle was rarely involved [3]. Here, we set up a new strategy to control the rotational kinetic of the wheel by introducing a "brake" on the wheel moiety, and demonstrated that the rotation kinetic of the wheel could be accurately manipulated by temperature variation.
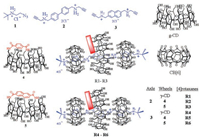
Cyclodextrins (CDs) are one of the most widely studied host molecules, bearing the advantage of good water solubility, inherent chirality, versatile binding ability and ready availability [4]. We have demonstrated that chemical modification could significantly improve the binding and chiral discrimination of CDs [5, 6]. Both CDs and curcubit[6]uril (CB[6]) have been applied as the wheels of rotaxanes due to their round shape and unique complexation ability with organic guests in aqueous solution. γ-CD-CB[6]-cowheeled [4]rotaxanes R1-R6 were synthesized by mixing γ-CD, CB[6], an alkynyl-terminated aromatic axle 2 or 3, and a bulky azide 1 together in aqueous solution at room temperature. In rotaxanes R2-R6, capped γ-CD were used as the wheel part. By introducing capping moiety, the size and shape of CDs as well as their complexation behavior could be significantly varied [7].
CB[6], 6A, 6X-dideoxy-6A, 6X-diamino-γ-CD and axle molecules were prepared according to the reported procedures [8]. The [4]rotaxanes were formed in good yield at room temperature as a result of CB[6]-catalyzed click-reaction [9]. We have demonstrated that the hydrogen bonding formed between CB[6] and CD played an important role in the efficient formation of [4]rotaxane [7b]. The rotaxanes R1-R3, containing an axle of biphenyl have been used as a supramolecular sensitizer for the chiral photoisomerization of cyclooctadiene. The capping part can further confine the orientational freedom of the axle part and therefore improved the reaction selectivity. It occurred to us that the capping moiety may also play a role of brake that can control the rotation kinetic of the axle. The steric hindrance effect between the cap and the axle molecule may slow down the rotation of the axle molecule along the axis of the CD cavity. Therefore, we investigated the NMR spectra of R1-R3 in water (Fig. 1). All biphenyl protons should stay in a different environment if the rotation of the biphenyl group was stopped due to the unsymmetrical structure caused by the introduction of capping moiety. However, for all these three rotaxanes, only one set of the 1H NMR spectra were observed, indicating that the biphenyl group in the cavity of γ-CD still can freely rotate on the NMR timescale. We ascribed the failure of braking to the relatively small size of biphenyl and the internal rotation of two benzene rings around the biphenyl linkage, and therefore focused our investigation on the rotaxanes R4-R6, in which the relatively bulky naphthalene severs as the axle (Scheme 1).

|
Download:
|
| Fig. 1. 1H NMR (400 MHz) of hetero[4]rotaxanes R1 (upper), R2 (middle) and R3 (lower) measured at 25 ℃ in D2O. | |
|
Download:
|
| Scheme 1. Chemical structure of wheels, axles and hetero[4]rotaxanes. | |
It turned out that the UV–vis spectra of rotaxanes R4-R6 are primarily the result of the UV–vis spectra of the capping moiety and the axle molecule (Fig. 2). This indicated that the axle part and the capping moiety had not significant electronic interaction at their ground states. R4 and R5 showed positive circular dichroism spectra at the 1Bb band of naphthalene (Fig. 3). According to the Kajtar's sector rule [10], these positive induced circular dichroism is consistent with the parallel orientation of the naphthalene axle along the longitudinal axis of the CD cavity. However, R6 exhibited strong exciton coupling type circular dichroism (ECCD), due to the close and chiral orientation of two naphthalenes. The positive ECCD signal (positive peak at 322 nm and a negative peak at 228 nm) indicated a right-handed helical arrangement of the capping moiety and the axle.

|
Download:
|
| Fig. 2. Fluorescence spectra of 1.0 μmol/L of 3 (black solid line), R4 (light blue dash dotted line), R5 (green dotted line) and R6 (red dash line) measured in water at 25 ℃, λex = 280 nm (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article). | |
|
Download:
|
| Fig. 3. 1H NMR (400 MHz) of hetero[4]rotaxanes R4 (upper), R5 (middle) and R6 (lower) measured at 25 ℃ in D2O. | |
The rotaxane R4, wheeled with native γ-CD, showed fluorescence spectrum similar to 3 with a higher intensity, presumably due to the protection of CD wall. On the contrary, R6 showed only the fluorescence of the capping moiety, and the fluorescence due to the axle moiety is negligible. We ascribed this to the extremely high efficiency of fluorescence resonant energy transfer from the axle moiety to the capping naphthalene as a result of the close interlocking of the two moieties. No excimer fluorescence could be seen with R6, for which the poor orbital overlap between two orthogonal naphthalenes should be responsible. Interestingly, a structureless broad fluorescence peaked at around 430 nm was observed with R5 in addition to the structural emission of the naphthalene axle (Fig. 2, green dotted line). We assigned the emission to the exciplex fluorescence formed between the naphthalene and the terephthalate. This is presumably due to that the terephthalate capping in R5 has a smaller size than the naphthalene capping in R6 and is, therefore, more flexible, which makes it easier forming π-π stacking with the axle.
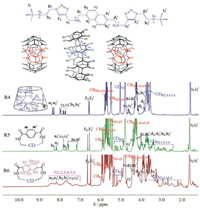
The 1H NMR spectra of rotaxanes R4-R6 were measured in D2O at 25 ℃. As shown in Fig. 3, all three rotaxanes showed two sets of CB[6]'s proton signals, as a result that two CB[6] positioned at the secondary and primary rims of γ-CD, respectively. The 1H NMR spectra of R4 exhibited one set of aromatic proton signals, and the H2, H3, H4, H5 and H6 proton signals of the γ-CD were congested in a narrow region of 3.4–3.8 ppm. This is reasonable as the large cavity of γ-CD should allow the naphthalene axle to freely rotate. For R5, the 1H NMR of CD exhibited a much wider distribution, giving an upfield shift up to 2.5 ppm. This indicated that the aromatic capping and the axle exerted unequal shielding and deshielding effects on each glucose unit of the CD. However, totally only one set of proton signals were seen for R5, suggesting that the rotation of the naphthalene axle is still fast on the NMR timescale.
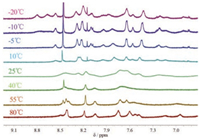
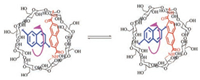
The 1H NMR spectra of R6 showed even significant upfield shift, with some CD protons shifted as far as 2.1 ppm (Fig. 3). Interestingly, the aromatic and CD proton signals of R6 were clearly broadened at 25 ℃, implying that the rotation of the axle was slow. To confirm this, the VT NMR of rotaxanes were performed in a mixture of D2O and CD3OD, which allowed us to measure the NMR spectra at a temperature lower than 0 ℃. As illustrated in Fig. 4, both R3 and R5 showed only one set of protons even at -20 ℃, suggesting a fast rotation of the wheel. Nevertheless, the proton signals became broad when lowering the temperature to -10 ℃ and -20 ℃. This observation raveled that the rotation speed slowed down with decreasing the temperature. In contrast, R6 showed broad and overlapped 1H NMR signals at 25 ℃ (Fig. 5), and raising the temperature to 40 ℃ led to an even broader and featureless 1H NMR spectrum. The broad and coalesced proton signals become sharper and differentiable with further increasing the temperature, to show a relatively clear 1H NMR spectrum at 55 ℃ and 80 ℃. Interestingly, the 1H NMR signals of R6 also became clear and sharp when lowering the temperature. The signals divided into two sets of proton signals at -20 ℃. The two sets of 1H NMR signals indicated that the naphthalene axle for R6, in which both the capping and the axle moieties constituted with relatively bulky naphthalene group, should cause strong steric interaction and therefore the two conformations of R6 interconvert slowly at low temperature (Scheme 2).

|
Download:
|
| Fig. 4. Partial 1H NMR spectra (600 MHz, D2O:CD3OD = 3:7) of hetero[4]rotaxane R3 (left) and R5 (right) recorded at -20 ℃, -10 ℃, -5 ℃, 10 ℃ and 25 ℃. | |
|
Download:
|
| Fig. 5. Partial 1H NMR (600 MHz, D2O:CD3OD = 3:7) spectra of R6 at -20 ℃, -10 ℃, -5 ℃, 10 ℃, 25 ℃, 40 ℃, 55 ℃ and 80 ℃. | |
|
Download:
|
| Scheme 2. Two rotational conformations of R6. | |
In summary, we have synthesized a series of CB[6] and γ-CD cowheeled [4]rotaxanes through a CB[6]-templated azide-alkyne 1, 3-dipolar cycloaddition. The aromatic capping moiety on the primary rim of γ-CD can interact with the aromatic axle at the ground and/or excited states. The rotaxanes having a naphthalene axle showed exciton coupling-type circular dichroism when wheeled with a naphthalene-capped γ-CD or exciplex fluorescence when wheeled with a terephthalate-capped γ-CD. Variable temperature 1H NMR proved that the rotation speed of the axle was affected by both the geometry of capping groups and the temperature. The rotaxane with naphthalene groups on the capping and axle moieties showed rotation kinetics slower than NMR timescale at low temperature, due to the steric interaction. The braking effect caused by capping groups in the present study established a new strategy to control the rotational kinetics of rotaxanes, and thus opens a new window for building intelligent molecular mechanical devices.
AcknowledgmentsWe acknowledge the support of this work by the National Natural Science Foundation of China (Nos. 21871194, 21572142, 21372165, 21402129 and 21402110), National Key Research and Development Program of China (No. 2017YFA0505903), Science & Technology Department of Sichuan Province (No. 2017SZ0021), and Comprehensive Training Platform of Specialized Laboratory, College of Chemistry, Sichuan university. The kind assistance on the fluorescence measurements from Prof. Peng Wu in Analytical & Testing Center, Sichuan University was greatly appreciated.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.12.002.
| [1] |
(a) V. Balzani, A. Credi, F.M. Raymo, J.F. Stoddart, Angew. Chem. Int. Ed. 39 (2000) 3348-3391; (b) J.P. Sauvage, C. Dietrich-Buchecker, Molecular Catenanes, Rotaxanes and Knots: A Journey Through the World of Molecular Topology, John Wiley & Sons, Weinheim, 1999. |
| [2] |
(a) N. Koumura, R.W. Zijlstra, R.A. Delden, et al., Nature 401 (1999) 152-155; (b) J. Conyard, A. Cnossen, W.R. Browne, et al., J. Am. Chem. Soc. 136 (2014) 9692-9700; (c) J.P. Collin, F. Durola, P. Mobian, et al., Eur. J. Inorg. Chem. (2007) 2420-2425. |
| [3] |
(a) E. Busseron, C. Romuald, F. Coutrot, Chem.-Eur. J. 16 (2010) 10062-10073; (b) T.R. Kelly, Acc. Chem. Res. 34 (2001) 514-522; (c) L.E. Harrington, L.S. Cahill, M.J. McGlinchey, Organometallics 23 (2004) 2884-2891; (d) D. Zhang, Q. Zhang, J.H. Su, et al., Chem. Commun. (2009) 1700-1702; (e) A. Faulkner, T.V. Leeuwen, B.L. Feringa, et al., J. Am. Chem. Soc. 138 (2016) 1359-1360; (f) W. Wu, S. Song, X. Cui, et al., Chin. Chem. Lett. 29 (2018) 95-98; (g) J. Yao, W. Wu, W. Liang, et al., Angew. Chem. Int. Ed. 56 (2017) 6869-6873. |
| [4] |
(a) Y. Wang, X. Qiao, W. Li, et al., Anal. Chim. Acta 650 (2009) 124-130; (b) W. Liang, C. Yang, M. Nishijima, et al., Beilstein J. Org. Chem. 8 (2012) 1305; (c) C. Yang, Q. Wang, M. Yamauchi, et al., Photochem. Photobiol. Sci. 13 (2014) 190-198; (d) X. Wei, W. Liang, W. Wu, et al., Org. Biomol. Chem. 13 (2015) 2905-2912; (e) J. Yi, W. Liang, X. Wei, et al., Chin. Chem. Lett. 29 (2018) 87-90. |
| [5] |
(a) Q. Wang, C. Yang, G. Fukuhara, et al., Beilstein J. Org. Chem. 7 (2011) 290; (b) C. Yang, C. Ke, F. Kahee, et al., Aust. J. Chem. 61 (2008) 565-568; (c) C. Yang, T. Mori, Y. Inoue, J. Org. Chem. 73 (2008) 5786-5794; (d) C. Yang, A. Nakamura, T. Wada, Y. Inoue, Org. Lett. 8 (2006) 3005-3008; (e) D.Q. Yuan, N. Kishikawa, C. Yang, et al., Chem. Commun. (2003) 416-417; (f) C. Yang, T. Mori, Y. Inoue, J. Org. Chem. 73 (2008) 5786-5794. |
| [6] |
(a) C. Yang, Chin. Chem. Lett. 24 (2013) 437-441; (b) Q. Huang, L. Jiang, W. Liang, et al., J. Org. Chem. 81 (2016) 3430-3434; (c) Q. Wang, C. Yang, C. Ke, et al., Chem. Commun. 47 (2011) 6849-6851; (d) X. Wei, W. Wu, R. Matsushita, et al., J. Am. Chem. Soc. 140 (2018) 3959-3974; (e) C. Yang, Y. Inoue, Chem. Soc. Rev. 43 (2014) 4123-4143; (f) J. Yao, Z. Yan, J. Ji, et al., J. Am. Chem. Soc. 136 (2014) 6916-6919; (g) J.C. Gui, Z.Q. Yan, Y. Peng, et al., Chin. Chem. Lett. 27 (2016) 1017-1021; (h) C. Yang, T. Mori, T. Wada, Y. Inoue, New J. Chem. 31 (2007) 697-702; (i) R. Lu, C. Yang, Y. Cao, et al., Chem. Commun. (2008) 374-376; (j) C. Yang, M. Nishijima, A. Nakamura, et al., Tetrahedron Lett. 48 (2007) 4357-4360. |
| [7] |
(a) C. Yang, H.K. Young, N. Selvapalam, et al., Org. Lett. 9 (2007) 4789-4792; (b) Z. Yan, Q. Huang, W. Liang, et al., Org. Lett. 19 (2017) 898-901; (c) L. Dai, W. Wu, W. Liang, et al., Chem. Commun. 54 (2018) 2643-2646. |
| [8] |
(a) S.Y. Jon, N. Selvapalam, D.H. Oh, et al., J. Am. Chem. Soc. 125 (2003) 10186-10187; (b) C. Yang, G. Fukuhara, A. Nakamura, et al., J. Photochem. Photobio. A: Chem. 173 (2005) 375-383; (c) C. Yang, A. Nakamura, T. Wada, Y. Inoue, Org. Lett. 8 (2006) 3005-3008. |
| [9] |
C. Ke, R.A. Smaldone, T. Kikuchi, H. Li, Angew. Chem. Int. Ed. 52 (2013) 381-387. DOI:10.1002/anie.201205087 |
| [10] |
M. Kajtár, C. Horváth-Toró, É. Kuthi, J. Szejtli, Proceedings of the First International Symposium on Cyclodextrins, Springer, 1982, pp. 181-193.
|
 2019, Vol. 30
2019, Vol. 30 

