Antibiotics are drugs of natural, semisynthetic or synthetic origin. They have been increasingly used for the treatment of bacterial diseases in humans and animals. In addition, these drugs are important in animal husbandry because they significantly enhance growth when added to animal feed. The extensive use of antibiotics has triggered the development of bacterial resistance [1], which, in recent years, has become an international public health issue [2]. Much attention has been paid to food-producing animals as a potential source of antibiotic-resistant bacteria in humans. As a result, antibiotic residues in edible animal products are of great concern to regulatory agencies and consumers.
In recent years, seven antibiotic families have been employed as veterinary drugs, of which sulfonamides and fluoroquinolones (FQs) have been the most used [3]. FQs, which are secondgeneration quinolone antibiotics, consist of a group of broadspectrum antibacterial agents with a unique mechanism of action and wide clinical use [4]. Many methods have been described for the determination of FQs such as microbiological assay [5, 6], immunoassay [7], time-resolved fluoroimmunoassay (TRIFIA) [8], fluorescence polarization immunoassay (FPIA) [9], spectrophotometry [10], enzyme-linked immunosorbent assay (ELISA) [11], high-performance liquid chromatography (HPLC) with diode array [12], fluorescence (FL) [13, 14], and mass spectrometry (MS) [15]. However, all of these techniques are time consuming and expensive, requiring complex laboratory equipment and trained personnel. Additionally, the methods require tedious samplepreparation procedures based on solid-phase extraction (SPE) and multistep clean-up. Therefore, reliable screening methods for rapid, selective and sensitive detection of trace FQ residues are necessary to ensure food safety.
Metal-organic coordination polymers (MOCPs), i.e., infinite coordination polymers (CPs) [16] or metal-organic frameworks (MOFs) [17], are formed by metal ions and organic ligands. MOCPs have been rapidly emerging as highly important functional materials. Due to their massive surface areas, tunable pore sizes, and high thermal stability, as well as attractive magnetic, electrical, optical, and catalytic properties, these materials have been exploited in many fields [18] such as the storage and separation of small molecules [19-21], molecular sieving [22], sensors [23, 24], medical imaging [25], drug delivery, and catalysis [26, 27]. Among MOCPs, lanthanide coordination polymer nanoparticles (Ln-CPs) have attracted considerable interest due to the unique optical properties of lanthanide ions. Because of their advantageous combination of permanent porosities and unique luminescent properties, Ln-CPs are superior to other types of materials for applications in luminescent chemical sensing and biomedical imaging as well as drug delivery monitoring and treatment [28]. However, a rapid screening method based on LnCPs for the detection of FQs in food samples has not been proposed until now.
In the present work, coordination polymer nanosheets (CPNSs) were prepared in a one-pot manner by mixing the solutions of Tb3+ and adenosine monophosphate (AMP). Based on the transmission electron microscopy (TEM), Fourier transform infrared (FTIR) spectroscopy and fluorescence spectroscopy results, the interaction mechanisms between Tb/AMP CPNSs and FQs were investigated in detail. ACPNS-based fluorescent sensor platform for the detection of the family of FQs in real milk samples was successfully developed.
Biomolecules possess the intrinsic property of self-assembly [29]. A nucleotide is a nucleobase, and in recent years, the biomolecule has been used to build MOCPs [30-32]. Here, Tb3+-based CPNSs were prepared by employing AMP as bridging ligands. Fig. 1a shows that the AMP/Tb CPNSs are a network structure composed of nanoscale fibers. After the addition of norfloxacin (Nor) (Fig. 1b), while the structure of AMP/Tb CPNSs remains unchanged, a remarkable cross-linking phenomenon is observed. These results indicate that the coordination network flexibly rearranged according to the molecular shapes of the guest molecules, supporting a key feature of adaptive self-assembly that the inclusion of guest molecules does not affect the morphology of host assemblies [33].

|
Download:
|
| Fig. 1. TEM images of AMP/Tb CPNSs (a) and AMP/Tb CPNSs incorporated with Nor (b). Scale bar: 200 nm. | |
Fig. S2 (Supporting information) shows the FTIR spectra of AMP/Tb in the absence and presence of FQs. Compared to those observed with AMP alone, the changes in the frequencies of the phosphate and FQ stretching vibrations with AMP/Tb indicate that both the phosphate and nucleobase moieties in AMP are involved in the coordination bonds [34]. After the incorporation of FQs, the disappearance of the C = O stretching vibrations peak of FQs at 1748 cm-1 and the appearance of the C-O stretching vibrations peak of FQs at 1074 cm-1 are found for the AMP/Tb CPNSs coexisting with FQs, implying the presence of a coordination interaction between Tb3+ and FQs. The shift in the phosphate signal frequency from 1097 cm-1 to 1109 cm-1 reflects the interaction of the AMP phosphate with Tb3+. Therefore, FQ involvement in the formation of the polymeric coordination network is speculated.
According to Fig. 2, unlike the case of Tb3+, AMP/Tb CPNSs show no fluorescence, confirming that AMP and Tb3+ undergo complexation. The Tb3+ luminescence is often quenched in water due to the deactivation of the excited states through the O-H vibrational modes of the coordinated water molecules [35]. However, with the addition of FQs, a significant emission of Tb3+ with peaks at 490, 545, 584, and 620 nm is observed when the excited wavelength is set at 284 nm. These peaks can be assigned to the energy transfer from FQs to Tb3+ which triggers the 5D4 to 7Fj (j = 3~6) Tb3+ electronic transitions [36]. Among these peaks, the emission at 545 nm is the strongest. Fig. 2 shows that the fluorescence intensity of AMP/Tb-FQs is stronger than the intensity of Tb3+ and Tb-FQs because Tb3+ is occupied by hydroxyl groups in the aqueous solution. After Tb3+ complexes with AMP, the steric hindrance effect restricts the occupation of these sites by –OH, increasing the fluoresent efficiency of the complexation of FQs with Tb3+.

|
Download:
|
| Fig. 2. Emission spectra of Tb, AMP-Tb, Tb-Nor and AMP-Tb-Nor in aqueous solution at the same concentration. Nor: 5.0 μmol/L. | |
Moreover, we investigated the fluorescent behaviors of AMP/Tb CPNSs in the dispersed and separated states. Green fluorescence is observed from the AMP/Tb CPNS suspension in the presence of FQs. However, after the AMP/Tb CPNSs-FQ suspension was centrifuged, only the precipitation of the suspension shows a green fluorescence, and no fluorescence is observed from the supernatant. The results suggest that FQs did not adsorb onto the external surface of the AMP/Tb CPNSs but did participate in the coordination of Tb3+, resulting in the formation of the AMP/Tb-FQ complexes.
The water solubility of CPNSs is determined by their surfacemodified hydrophilic functional group, i.e., phosphate. Phosphate is involved in the synthesis of AMP and Tb, causing AMP/Tb CPNSs to be insoluble in water and to form a suspension. FQs are amphoteric substances containing carboxyl acid and amino groups. With the addition of FQs, the carboxyl acid and keto groups of FQs combine with AMP/Tb CPNSs while the piperazinyl group stretches out with a positive charge, changing the solubility of the CPNSs. Thus, after adding excessive FQs, the suspension becomes transparent. This phenomenon further supports the occurrence of the chemical coordination between FQs and Tb3+ on the surface of AMP/Tb CPNSs. This result suggests that the binding site of AMP and Tb3+ is at the phosphate site rather than through the nitrogen atoms, as claimed by Tan and coworkers [32].
To further investigate the effect of AMP-Tb CPNSs on the fluorescence behavior of each FQ, AMP/Tb was reacted with various FQs at the same concentration. Each FQ can bind to AMP/Tb CPNSs, but their fluorescence intensities are not identical (Fig. S3 in Supporting information). First, the reactions of two structurally similar FQs, Nor and pefloxacin (Pef), with AMP/Tb CPNSs are found to generate similar fluorescence intensities. Furthermore, when sparfloxacin (Spa) and fleroxacin (Fle) were combined with AMP-Tb CPNSs, the results indicate that the fluorescence intensities of the two quinolones are slightly lower than those of Nor and Pef, however, that of Fle is the weakest. This phenomenon reminds us of the particular structure of Fle, where electron-accepting F atoms are present at the 1-, 6-, and 8- positions. Although Spa also has electron-accepting groups at the 6- and 8- positions, the electron-donating cyclopropyl group in the 1-position replaces F in the 1-position of Fle, and the electron-donating -NH2 group appears in the 5-position.
To verify the hypothesis that these electron-donating and electron-accepting groups affect the combination of FQs and AMPTb CPNSs, nalidixic acid (Nal) was reacted with AMP-Tb CPNSs. The results indicate that the fluorescence intensity of Nal is substantially higher than that of Nor, which is related to the electrondonating -CH2-CH3 group in the 1-position of Nal. Moreover, the fluorescence intensity of CPNS-flumequine (Flu) is only slightly lower than that of Nal due to the addition of an electron-accepting group to the 6-position of Flu.
Hence, two factors affect the combination of FQs and AMP/Tb CPNSs. The first factor is the presence of electron-donating and electron-accepting groups on the FQ structure itself. In the presence of electron-accepting groups (such as F), the conjugation of FQs with AMP/Tb CPNSs is inhibited, where as the presence of electron-donating groups (such as -CH2-CH3) may promote the conjugation of FQs with AMP/Tb CPNSs. Second, due to the steric hindrance effect, the piperazine ring at the 7-position of the FQ restricts the conjugation of FQs with AMP/Tb CPNSs. The schematic illustration of the present CPNSs for the detection of FQs was shown in Scheme 1.

|
Download:
|
| Scheme 1. The turn-on fluorescence mechanisms of CPNSs for the detection of Nor. | |
AMP disodium, guanosine monophosphate (GMP) disodium, cytidine monophosphate (CMP) disodium and uridine monophosphate (UMP) disodium were all investigated as coordinators of Tb3+. The results show that both AMP and GMP can react with Tb3+ to form CPNSs and conjugate with FQs to achieve their quantitative detection. GMP/Tb CPNSs exhibit a fluorescent response at the characteristic emission wavelength of Tb3+. We attribute this to the transfer of energy from the guanine base to the Tb3+ ion emitting from the 5D4 state; this transfer is facilitated by the coordination of the Tb3+ ions to the oxygen at the 6-position and to the nitrogen at the 7-position [34]. Due to steric hindrance, the reaction between FQs and GMP/Tb CPNSs becomes more difficult. Thus, AMP is selected as the component of the self-assembly with Tb3+.
In addition, the fluorescence of AMP/Tb-FQ complexes is strongly affected by the pH of the reaction medium. The fluorescence intensity of the AMP/Tb-FQ complexes is enhanced as the pH is increased from 7.1 to 7.5, and the highest enhancement is observed at pH 7.5. This fluorescence enhancement may be ascribed to the deprotonation of AMP and FQs in the neutral environment that promotes the formation of the AMP/Tb-FQ complexes. However, a gradual decrease in the fluorescence intensity of the AMP/Tb-FQ complexes is observed when the pH value is greater than 7.5. Under strongly basic conditions, the majority of FQs show a remarkable decrease in fluorescence intensity, further influencing the energy transfer between the FQs and Tb3+, and this quenching behavior may be related to the existing forms of the FQ species.
The effects of the terbium ion to AMP molar ratio on the luminescence of the coordination polymer were investigated. The luminescence intensity first increases with the amount of AMP and then decreases (data not shown). The maximum luminescence intensity of the coordination polymer is observed for the AMP toTb molar ratio of 1:1.
To evaluate the performance of AMP/Tb as a nanosensor toward FQ detection, FQs in different concentrations were added to the AMP/Tb solution to measure the fluorescence responses under the abovementioned optimal experimental conditions. As shown in Fig. 3, the fluorescence intensity of the Tb3+ emission at 545 nm is very sensitive and increases gradually with the FQ concentration, revealing that FQs strongly coordinated with the Tb3+ center to replace H2O [37]. The F545nm increases linearly with the FQ concentration (Fig. S4 in Supporting information).
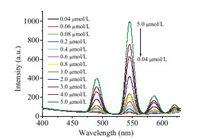
|
Download:
|
| Fig. 3. Fluorescence responses of AMP-Tb CPNSs upon the addition of Nor in various concentrations (0.04 - 5.0 μmol/L). | |
The analytical figures of merit obtained under the optimal conditions described above are summarized in Table S1 (Supporting information). The calibration curves were obtained using the standard solutions of the FQs and covered the entire linear range; the data for each point were obtained in triplicate.
A good linear relationship (R2 > 0.99) is obtained in the appropriate range. The limits of detection (LODs) and the slopes of the regression equations for all studied FQs are very similar (Table S1). The precision and stability of the proposed method were studied by assaying the standard solutions of FQs (0.1 μmol/L). The relative standard deviation (RSD) is 0.78% within a day (n = 9) in all cases.
To evaluate the accuracy of the proposed method for the determination of the FQs in milk samples purchased from the supermarket, recovery studies using Nor as the model compound were carried out on real samples to which known amounts of drugs were added. The results of the recovery tests are listed in Table S2 (Supporting information). The intraday recoveries for the target FQs are between 94.90% and 108.24%, and the interday recoveries are between 96.41% and 103.84%. The recoveries are satisfactory for the determination of FQs at such trace levels, and thus, the proposed method is found to be applicable for the detection of FQ residues in milk.
Table S3 (Supporting information) summarizes the time required for different methods for the determination of FQs. The detection process of the present system is simpler than those for the FLU [38-40], TRIFIA [8], FPIA [9, 18], and ELISA [41, 42] detection methods.
The results demonstrate that the proposed method offers a rapid approach for the detection of FQs. The present method based on AMP/Tb CPNSs exhibits the advantages of a direct and rapid detection procedure, simple sample pretreatment processes and excellent stability and selectivity. To the best of our knowledge, the present work represents the first use of an Ln-CP nanosensor for the detection of total FQs in milk. The proposed strategy might provide a new platform for rapid fluorescent detection of antibiotics based on lanthanide coordination polymer nanomaterials.
AcknowledgmentsThe authors would like to thank for the financial supports from the National Natural Science Foundation of China (Nos. 81760601, 21265013 and 81260435), the Natural Science Foundation of Jiangxi Province (No. 20171BAB215050), and the Innovation Fund Designated for Graduate Students of Jiangxi Province (No. YC2017- S085).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.11.026.
| [1] |
G. Bell, C. MacLean, Trends Microbiol. 26 (2018) 471-483. DOI:10.1016/j.tim.2017.11.005 |
| [2] |
M. Ferri, E. Ranucci, P. Romagnoli, V. Giaccone, Crit. Rev. Food Sci. Nutr. 57 (2017) 2857-2876. DOI:10.1080/10408398.2015.1077192 |
| [3] |
C. Cháfer-Pericás, Maquieira Á., R. Puchades, TrAC Trends Anal. Chem. 29 (2010) 1038-1049. DOI:10.1016/j.trac.2010.06.004 |
| [4] |
K. Drlica, A. Mustaev, T. Towle, L. Gan, R. Kerns, ACS Chem. Biol. 9 (2014) 2895-2904. DOI:10.1021/cb500629k |
| [5] |
M. Tumini, O. Nagel, M.P. Molina, R. Althaus, Int. Dairy J. 64 (2017) 9-13. DOI:10.1016/j.idairyj.2016.08.008 |
| [6] |
A.S. Wagman, R. Cirz, G. Mcenroe, et al., ChemMedChem 12 (2017) 1687-1692. DOI:10.1002/cmdc.201700426 |
| [7] |
H. Ni, S. Zhang, X. Ding, et al., Anal. Lett. 47 (2014) 2844-2856. DOI:10.1080/00032719.2014.924009 |
| [8] |
Z. Bin, Z. Kai, Z. Jue, et al., Toxicol. Mech. Method 23 (2013) 323-328. DOI:10.3109/15376516.2012.757685 |
| [9] |
J. Chen, I.A. Shanin, S. Lv, et al., J. Sci. Food Agric. 96 (2016) 1341-1346. DOI:10.1002/jsfa.2016.96.issue-4 |
| [10] |
B.G. Gowda, J. Seetharamappa, Anal. Sci. 19 (2003) 461-464. DOI:10.2116/analsci.19.461 |
| [11] |
Z. Liu, S. Lu, C. Zhao, et al., J. Sci. Food Agric. 89 (2009) 1115-1121. DOI:10.1002/jsfa.v89:7 |
| [12] |
H.B. Zheng, J.Z. Mo, Y. Zhang, et al., J. Chromatogr. A 1329 (2014) 17-23. DOI:10.1016/j.chroma.2013.12.083 |
| [13] |
A. Pochivalov, I. Timofeeva, C. Vakh, A. Bulatov, Anal. Chim. Acta (2017) 35-44. |
| [14] |
R. Duan, J. Jiang, S. Liu, et al., J. Sci. Food Agric. 97 (2017) 2569-2574. DOI:10.1002/jsfa.2017.97.issue-8 |
| [15] |
J.L. Urraca, M. Castellari, C.A. Barrios, M.C. Moreno-Bondi, J. Chromatogr. A 1343 (2014) 1-9. DOI:10.1016/j.chroma.2014.03.045 |
| [16] |
A.M. Spokoyny, D. Kim, A. Sumrein, C.A. Mirkin, Chem. Soc. Rev. 40 (2009) 1218-1227. |
| [17] |
A. Schneemann, V. Bon, I. Schwedler, et al., Chem. Soc. Rev. 43 (2014) 6062-6096. DOI:10.1039/C4CS00101J |
| [18] |
B. Li, B.L. Chen, Porous Lanthanide Metal-Organic Frameworks for Gas Storage and Separation, in: P. Cheng (Ed.), Lanthanide Metal-Organic Frameworks, Springer-Verlag, Berlin, Berlin, 2015, pp. 75-107.
|
| [19] |
Q.Q. Li, J.G. Duan, W.Q. Jin, Chin. Chem. Lett. 29 (2018) 854-856. DOI:10.1016/j.cclet.2017.11.008 |
| [20] |
W.D. Fan, Y.T. Wang, Z.Y. Xiao, et al., Chin. Chem. Lett. 29 (2018) 865-868. DOI:10.1016/j.cclet.2017.11.020 |
| [21] |
S. Yang, X. Lin, A.J. Blake, et al., Nat. Chem. 1 (2009) 487-493. DOI:10.1038/nchem.333 |
| [22] |
L.J. Murray, M. Dinca, J.R. Long, Chem. Soc. Rev. 38 (2009) 1294-1314. DOI:10.1039/b802256a |
| [23] |
K. Chen, C.D. Wu, Chin. Chem. Lett. 29 (2018) 823-826. DOI:10.1016/j.cclet.2017.09.040 |
| [24] |
B. Liu, Y. Huang, Q. Shen, et al., RSC Adv. 6 (2016) 100743-100747. DOI:10.1039/C6RA20357D |
| [25] |
W. Morris, W.E. Briley, E. Auyeung, M.D. Cabezas, C.A. Mirkin, J. Am. Chem. Soc. 136 (2014) 7261-7264. DOI:10.1021/ja503215w |
| [26] |
L. Ma, J.M. Falkowski, C. Abney, W. Lin, Nat. Chem. 2 (2010) 838-846. DOI:10.1038/nchem.738 |
| [27] |
F. Song, C. Wang, J.M. Falkowski, L. Ma, W. Lin, J. Am. Chem. Soc. 132 (2010) 15390-15398. DOI:10.1021/ja1069773 |
| [28] |
B. Li, H.M. Wen, Y. Cui, G. Qian, B. Chen, Prog. Polym. Sci. 48 (2015) 40-84. DOI:10.1016/j.progpolymsci.2015.04.008 |
| [29] |
I. Imaz, M. Rubio-Martínez, J. An, et al., Chem. Commun. 47 (2011) 7287-7302. DOI:10.1039/c1cc11202c |
| [30] |
Y. Song, J. Chen, D. Hu, et al., Sensor. Actuat. B:Chem. 221 (2015) 586-592. DOI:10.1016/j.snb.2015.07.008 |
| [31] |
H. Tan, B. Liu, Y. Chen, J. Phys. Chem. C 116 (2012) 2292-2296. |
| [32] |
H. Tan, L. Zhang, C. Ma, et al., ACS Appl. Mat. Interfaces 5 (2013) 11791-11796. DOI:10.1021/am403442q |
| [33] |
C. Aimé, R. Nishiyabu, R. Gondo, N. Kimizuka, Chem. Eur. J. 16 (2010) 3604-3607. DOI:10.1002/chem.v16:12 |
| [34] |
R. Nishiyabu, N. Hashimoto, T. Cho, J. Am. Chem. Soc. 6 (2009) 2151-2158. |
| [35] |
J.C.G. Bünzli, C. Piguet, Chem. Soc. Rev. 34 (2005) 1048-1077. DOI:10.1039/b406082m |
| [36] |
F. Richardson, Chem. Rev. 82 (1982) 541-552. DOI:10.1021/cr00051a004 |
| [37] |
A.C. Ferrand, D. Imbert, A.S. Chauvin, et al., Chem. Eur. J. 13 (2007) 8678-8687. DOI:10.1002/(ISSN)1521-3765 |
| [38] |
J. Hua, Y. Jiao, M. Wang, Y Yang, Microchim. Acta 185 (2018) 137. DOI:10.1007/s00604-018-2685-x |
| [39] |
R. Mirzajani, N. Pourreza, J. Burromandpiroze, Ultrason. Sonochem. 40 (2018) 101-112. DOI:10.1016/j.ultsonch.2017.06.027 |
| [40] |
B. Mao, F. Qu, S. Zhu, J. You, Sensor. Actuat. B:Chem. 234 (2016) 338-344. DOI:10.1016/j.snb.2016.04.174 |
| [41] |
G.Y. Fan, R.S. Yang, J.Q. Jiang, et al., J. Zhejiang Univ. Sci. B 13 (2012) 545-554. DOI:10.1631/jzus.B1200001 |
| [42] |
M.S. Ha, M.S. Chung, D.H. Bae, Food Addit. Contam. A 33 (2016) 817-823. DOI:10.1080/19440049.2016.1179560 |
 2019, Vol. 30
2019, Vol. 30 

