b College of Energy and Environment Science, Yunnan Normal University, Kunming 650500, China;
c College of Chemical Science and Technology, Yunnan University, Kunming 650091, China
It is well known that sulfur dioxide (SO2) is one of the most common gaseous environmental pollutants [1-3], with the combustion of coal and fossil fuels, human exposure to SO2 has become increasingly widespread and causes mortal threats to human health. Epidemiological studies suggest that SO2 exposure not only induces many respiratory responses [4], but is also linked to lung cancer, cardiovascular diseases, and many neurological disorders, such as migraine headaches, stroke, and brain cancer [5]. These impacts could be ascribed to sulfite (SO32-) or bisulfite (HSO3-), which are the main existence forms of SO2 in aqueous solution [6]. Between these anions, HSO3- has been widely used as an antimicrobial agent, enzyme inhibitor and antioxidant for foods, beverages and pharmaceutical products [7]. Despite its valuable properties, HSO3- has harmful effects on tissue, cells, and biomacromolecules. Due to toxicity and exposure to high levels of HSO3- inducing diseases in some individuals, such as allergic reaction, asthma, gastrointestinal, and skin allergy diseases [8], its use in food is strictly controlled [9, 10]. Therefore, the development of rapid, facile, and reliable detection techniques for HSO3- is crucial protecting human life.
Fluorescence imaging is a sensitive, rapid, and efficient bioanalytical tool for tracing the state, changes, and activities of targets in vivo, thereby facilitating progress in the fields of cell biology and imaging in therapeutics [11, 12]. In recent years, fluorescent probes have received a great deal of attention due to their potential applications in environmental chemistry, biology, and medicine through fluorescence sensing and recognition of anions by receptors [13-15]. At present a large number of fluorescent probes for HSO3- have been reported, with only a few groups having developed ratiometric fluorescent sensors for the rapid detection of HSO3- in living cells. For example, Wu et al. reported a water-soluble near-infrared probe for colorimetric and ratiometric sensing of SO2 [16]. Xu et al. reported a mitochondriatargeted ratiometric fluorescent probe for the rapid, sensitive and specific detection of biological SO2 [17]. Ma et al. reported the ratiometric cell imaging of HSO3- inspired by the confined-space based FRET system [18]. Our work examines a ratiometric fluorescent Probe 1 (Scheme 1) for the selective detection of HSO3- based on the 1, 4-nucleophilic addition reaction with a carbazole as an electron donor (D) and an aldehyde as an electron acceptor (A). Probe 1 is composed of a hemicyanine and carbazole conjugate allowing for an increased conjugation system for cycloaddition. Through mass spectrometry analysis and the work curve, we found that the 1, 4-nucleophilic addition reaction occurred between the double bond and HSO3- resulting in a fluorescence emission change. Probe 1 was tested in the buffer solution of DMF:PBS (3/7, v/v, PBS 0.01 mol/L, pH 7.4), the probe exhibited high sensitivity and selectivity for HSO3-. In the maximum of fluorescence emission spectra a blue-shift from 570 nm to 445 nm was observed and the color of the solution changed from yellow to blue with the addition of HSO3- to a physiological aqueous solution. The high sensitivity and selectivity of Probe 1 permitted the detection of exogenous HSO3- in Hela cells and C. elegans [19, 20].

|
Download:
|
| Scheme 1. Probe 1 for fluorescent detection of HSO3-. | |
First of all, the materials used in this work were obtained from commercial suppliers and used without further purification unless otherwise noted. Flash chromatography was carried out on silica gel (200 - 300 mesh). 1H NMR spectra was recorded using 500 MHz and 13C NMR was recorded using 101 MHz. Chemical shifts were expressed in ppm and coupling constants (J) in Hz. Mass spectrometry was recorded with Xevo TQ-S and Q-TOF mass spectrometer. UV–vis spectra were recorded on U3010 spectrometer. Fluorescence spectra were recorded on F4500 spectrometer. Cell imaging was acquired on a Nikon ECLIPSE 90i confocal laser scanning microscope.
The Probe 1 stock solution (1 mmol/L) was prepared in DMF [21]. The concentration of Probe 1 in a working solution was 2 ×10-5 mol/L. Solutions of various testing species (SO42-, HCO3-, N3-, AcO-, SCN-, C2O42-, HPO42-, HSO3-, NO2-, F-, Cl-, Br-, I-, Cys, Hcy, GSH, S2-, SO32-) were prepared in distilled water (0.01 mol/L). The resulting solution was placed in a glass and quartz cuvette of 1 cm × 1 cm optical path length each time and the UV–vis or fluorescent spectra titrations were recorded. All spectroscopic experiments were carried out at room temperature.
HeLa cells were kindly provided by the Institute of Life Science at Yunnan University. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) containing 10% fetal bovine serum (FBS) at 37 ℃ in a 5% CO2 and 95% air incubator. The cells were incubated for 24 h before use, the cells were incubated with 10.0 mmol/L Probe 1 in a 5% CO2 and 95% air incubator for 30 min. Then, the cells were incubated with different concentrations of HSO3- in a 5% CO2 for 2 h, washed twice with PBS. Images were taken with an Olympus FV1000 confocal microscope with a 40× objective lens.
The wild-type strain of C. elegans was acquired from the Institute of Life Science at Yunnan University. The C. elegans at the fourth larval stage were incubated in Petri dishes filled with M9 buffer containing 20 μmol/L Probe 1 for 1 h at 20℃. After the incubation, the exposed nematodes were collected with a centrifugation at the speed of 3000 rpm for 2 min, followed by three times washing with M9 buffer. The nematodes were next incubated with increasing concentrations of HSO3- for 2 h at room temperature.
Precursor 2 was prepared according to a previously reported method [22]. Probe 1 was readily prepared by the condensation of compound 3 with Precursor 2 in the presence of piperidine and EtOH, giving Probe 1 in moderate yield (Scheme S1 in Supporting information). The structure of Probe 1 was confirmed by 1H NMR and 13C NMR, and HR-MS analysis. More detailed synthetic processes and structure characterizations can be found in Supporting information.
Next, with Probe 1 in hand, its spectral properties in the presence or absence of HSO3- in DMF:PBS (3/7, v/v, PBS 0.01 mol/L, pH 7.4) was evaluated. Probe 1 displayed a broad absorption band at 467 nm. Upon increasing the concentration of HSO3-, the band at 467 nm gradually decreased and a new absorption bands at 282 nm (ca.185 nm blue-shift) emerged with a well-defined isobestic point at 365 nm (Fig. 1a), as well as a noticeable color change from yellow to colorless (Fig. 1a inset). The significant colorimetric change was due to the interruption of the π- conjugation by HSO3-. By varying the concentrations of HSO3-, the ratio between the fluorescence spectra increment and the compound action was determined at 445 nm with a 1:1 (Probe 1: HSO3-) binding ratio (Fig. S1 in Supporting information). Subsequently, upon increasing the amount of HSO3-, the fluorescence emission band at 570 nm decreased dramatically, which lead to an increase in the band at 445 nm (Fig. 1b). The fluorescence intensity of Probe 1 in solution did not change, until the concentration of HSO3- reached 2 equiv. resulting in the color changing from yellow to blue (Fig. 1b inset). This important colorimetric change confirms the utilization of Probe 1 in the ratiometric detection of HSO3-. By plotting the fluorescence intensity (445 nm) against the concentration of HSO3-, a good linear relationship was obtained with the concentration ranging from 0 to 5 μmol/L. The limit of detection was calculated as 1.29 μmol/L (Fig. S2 in Supporting information).

|
Download:
|
| Fig. 1. UV–vis absorption (a) and fluorescence spectra (b) of probe 1 (2 ×10-5 mol/L) in the presence or absence of HSO3- (0 - 2 equiv.) in DMF:PBS (3/7, v/v, PBS 0.01 mol/L, pH 7.4). The inset shows color changes (a) and fluorescence changes (b) of probe 1 with HSO3-. | |
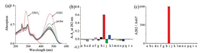
In order to prove that Probe 1 possesses high selectivity towards HSO3-, we investigated its response with the addition of various interferential species (Probe 1 only, SO42-, HCO3-, N3-, AcO-, SCN-, C2O42-, HPO42-, HSO3-, NO2-, F-, Cl-, Br-, I-, Cys, Hcy, GSH, S2-, SO32-) in DMF:PBS (3/7, v/v, PBS 0.01 mol/L, pH 7.4). As shown in Fig. 2a, Probe 1 began with ratiometric absorption peaks at 467 nm and 282 nm. When we added 2 equiv. of the various species to a solution of Probe 1, the absorption peak at 282 nm remained strong. In the case of GSH, an intense absorption peak was produced at 481 nm. Fig. 2b showed the accumulated data gathered for the effect the various species has on the 282 nm band. It was determined that the addition of other anionic species only gives a weak absorption change compared to the addition of HSO3-, which possesses a greater effect. We also examined the ratio of the absorption intensity at 282 nm and 467 nm (Fig. 2c), only with HSO3- showing the largest absorption intensity. This result highlights the high selectivity Probe 1 has towards HSO3-.
|
Download:
|
| Fig. 2. (a) Absorption spectra, (b) histogram of the enhanced value at 282 nm, (c) ratio changes of 282 nm and 467 nm of probe 1 (2 ×10-5 mol/L) in the presence of 2 equiv. various interferential species (a: probe 1 only, b- s: SO42-, HCO3-, N3-, AcO-, SCN-, C2O42-, HPO42-, HSO3-, NO2-, F-, Cl-, Br-, I-, Cys, Hcy, GSH, S2-, SO32-) in DMF:PBS (3/7, v/v, PBS 0.01 mol/L, pH 7.4). | |
Gratifyingly only when Probe 1 was treated with HSO3-, a significant change in the fluorescence emission at 445 nm was observed, compared to other species which had a much weaker effect (Fig. S3 in Supporting information). Even some interfering species showed certain responses towards HSO3-, their fluorescence changes were weak and cannot disturb the sensing of Probe 1. The experimental results showed that Probe 1 can be used to detect sulfites even in the presence of other interfering species.
In accordance with the UV-vis absorption spectra and fluorescence spectra, we observed that Probe 1 also has certain absorption and emission changes for GSH, Cys and Hcy. This was due to the presence of the aldehyde group in Probe 1, and GSH, Cys, and Hcy can thus interact with this group promoting spectrum changes. To a solution of Probe 1 (2 ×10-5 mol/L) in DMF:PBS (3/7, v/v, PBS 0.01 mol/L, pH 7.4), different concentrations of Cys, GSH and Hcy were added, as shown in Fig. S4 (Supporting information). Addition of Cys to the Probe 1 solution gave bands at approximate 430 nm and 573 nm (Fig. S4a). In the case of GSH and Hcy, two new fluorescence emission peaks were found at approximate 431 nm and 574 nm (Figs. S4b and S4c). Although Probe 1 displayed absorption and fluorescence emission for Cys, GSH, Hcy, the maximum absorption peak and maximum emission peak were different and much less intense compared to HSO3-, further highlighting the selectivity of Probe 1.
Furthermore, in order to verify the reaction mechanism of the Probe 1 and HSO3-, we investigated the mass spectrometry (MS) change of Probe 1 after the addition of HSO3-. Firstly, the MS analysis of Probe 1 gave m/z 421.23 ([M]+). After Probe 1 (0.5 mg) and HSO3- (1.0 mg) were stirred in DMF:PBS (3/7, v/v, PBS 0.01 mol/L, pH 7.4) for 1 h, the resulting MS showed a significant m/z value at 525 (calcd. 525.18) corresponding to the addition of HSO3- to Probe 1 [M + Na]+ (Fig. S5 in Supporting information). Therefore, we suggested that the vinyl group, rather than the aldehyde group of Probe 1 undergoes 1, 4-nucleophilic addition with HSO3- (Scheme S2 in Supporting information). In Fig. S6 (Supporting information), Probe 1 worked as a fast-response probe for HSO3- within 2 min. The high reactivity of Probe 1 and HSO3- showed that Probe 1 is suitable for the real-time monitoring of the HSO3- level in cells [23-25].
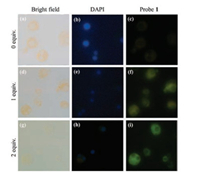
To investigate the capacity of the Probe 1 to fluorescence imaging in vivo, HeLa cells were treated with the probe and DAPI, a commercial nuclei dye. First, we stained the nucleus with DAPI and then incubated the cells with different concentrations of HSO3-. As seen in Fig. 3, after the addition of HSO3- to Probe 1, the cytoplasmic green fluorescence increased with an increase in HSO3- concentration in the green channel. The results indicated that Probe 1 can penetrate the cell membrane and trace the HSO3- in cytoplasmic part.
|
Download:
|
| Fig. 3. Confocal microscopy images of HeLa cells. (a - c) Probe 1 (20 μmol/L) only. (d - f) Probe 1 (20 μmol/L) and HSO3- (1 equiv.). (g - i) Probe 1 (20 μmol/L) and HSO3- (2 equiv.). (a), (d) and (g) are bright field images. (b), (e) and (h) are stained with DAPI (blue channel). (c), (f) and (i) are stained with probe 1 (green channel) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article). | |
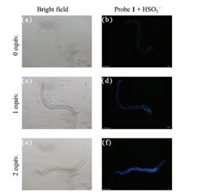
As well as HeLa cells, C. elegans was also examined. After incubation with Probe 1, a change in the fluorescence was detected by fluorescence microscopy (Fig. 4). No signal was observed in the nematodes pre-treated with 20 μmol/L Probe 1 for 2 h. With an increase in HSO3- concentration a blue fluorescence was emitted from the intestine and gonads of nematodes. This demonstrates the feasibility of employing Probe 1 for the detection of exogenous HSO3- in C. elegans.
|
Download:
|
| Fig. 4. Bright field images (a, c, e) and fluorescent images (b, d, f) for HSO3- in C. elegans. (a, b) Probe 1 (20 μmol/L) only. (b, d) Probe 1 (20 μmol/L) and HSO3- (1 equiv.). (e, f) Probe 1 (20 μmol/L) and HSO3- (2 equiv.). | |
In conclusion, we have reported a broadly emitting ratiometric fluorescent probe for the detection of the important HSO3- based on the 1, 4-nucleophilic addition reaction. Probe 1 displays high selectivity and sensitivity towards HSO3- detection. Furthermore, Probe 1 was found to be capable of detecting exogenous HSO3- in Hela cells and C. elegans. These results demonstrate that Probe 1 can be used to monitor the level of intracellular HSO3- in ratiometric fluorescence imaging. Our work holds great promise for the detection of HSO3- in biological systems, we hope that the synthesis of new probes in subsequent work will be of great significance in biomedical and clinical diagnostics.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21262050, 21302165, 21867019 and 41561108), the Foundation of the Department of Science and Technology of Yunnan Province (Nos. 2014HB008, 2017HB019).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.11.020.
| [1] |
H. Mana, U. Spohn, Anal. Chem. 73 (2001) 3187-3192. DOI:10.1021/ac001049q |
| [2] |
G.J. Mohr, Chem. Commun. 22 (2002) 2646-2647. |
| [3] |
M. Zhao, D.B. Hibbert, J.J. Gooding, Anal. Chim. Acta 556 (2006) 195-200. DOI:10.1016/j.aca.2005.05.083 |
| [4] |
S. Iwasawa, Y. Kikuchi, Y. Nishiwaki, et al., J. Occup. Health 51 (2009) 38-47. DOI:10.1539/joh.L8075 |
| [5] |
N. Sang, Y. Yun, H. Li, et al., Toxicol. Sci. 114 (2010) 226-236. DOI:10.1093/toxsci/kfq010 |
| [6] |
D.P. Li, X.J. Han, Z.Q. Yan, et al., Dyes Pigm. 151 (2018) 95-101. DOI:10.1016/j.dyepig.2017.12.056 |
| [7] |
A. Isaac, J. Davis, C. Livingstone, et al., Trends Analyt. Chem. 25 (2006) 589-598. DOI:10.1016/j.trac.2006.04.001 |
| [8] |
J.B. Chao, H.J. Wang, Y.B. Zhang, et al., New J. Chem. 42 (2018) 3322-3333. DOI:10.1039/C7NJ03903D |
| [9] |
T. Fazio, C.R. Warner, Food Addit. Contam. 7 (1990) 433-454. DOI:10.1080/02652039009373907 |
| [10] |
R.C. Claudia, J.C. Francisco, Food Control 21 (2010) 1331-1337. DOI:10.1016/j.foodcont.2010.04.005 |
| [11] |
D. Yang, H.Y. Tian, T.N. Zang, et al., Sci. Rep 7 (2017) 9174. DOI:10.1038/s41598-017-09525-2 |
| [12] |
L. Song, X.D. Sun, Y.H. Yao, et al., Chin. Chem. Lett. 27 (2016) 1776-1780. DOI:10.1016/j.cclet.2016.05.007 |
| [13] |
L. Yu, Y.M. Qiao, L.X. Miao, et al., Chin. Chem. Lett. 29 (2018) 1545-1559. DOI:10.1016/j.cclet.2018.09.005 |
| [14] |
P.A. Gale, G.S.E. Garcia, J. Garric, Chem. Soc. Rev. 37 (2008) 151-190. DOI:10.1039/B715825D |
| [15] |
M.R. Martinez, F. Sancenon, Chem. Rev. 103 (2003) 4419-4476. DOI:10.1021/cr010421e |
| [16] |
M.Y. Wu, K. Li, C.Y. Li, et al., Chem. Commun. (Camb.) 50 (2014) 183-185. DOI:10.1039/C3CC46468G |
| [17] |
J.C. Xu, J. Pan, X.M. Jiang, et al., Biosens. Bioelectron. 77 (2016) 725-732. DOI:10.1016/j.bios.2015.10.049 |
| [18] |
W.J. Ma, J.J. Du, J. Yin, et al., Sens. Actuators B-Chem. 267 (2018) 104-110. DOI:10.1016/j.snb.2018.03.184 |
| [19] |
Y. Liu, M.Y. Wu, Y.H. Liu, et al., Chem. Commun. 51 (2015) 10236-10239. DOI:10.1039/C5CC03055B |
| [20] |
Y. Liu, K. Li, K.X. Xie, et al., Chem. Commun. 52 (2016) 3430-3433. DOI:10.1039/C5CC10505F |
| [21] |
H. Wang, D. Yang, R. Tan, et al., Sens. Actuators B-Chem. 247 (2017) 883-888. DOI:10.1016/j.snb.2017.03.030 |
| [22] |
B. Jin, X. Zhang, W. Zheng, et al., Anal. Chem. 86 (2014) 943-952. DOI:10.1021/ac403676x |
| [23] |
Y. Zhang, Y.Y. Fu, D.F. Zhu, et al., Chin. Chem. Lett. 27 (2016) 1429-1436. DOI:10.1016/j.cclet.2016.05.019 |
| [24] |
L. Feng, M. Chhabra, W.H. So, et al., Chin. Chem. Lett. 29 (2018) 1147-1150. DOI:10.1016/j.cclet.2018.05.031 |
| [25] |
J.C. Xu, H.Q. Yuan, L.T. Zeng, et al., Chin. Chem. Lett. 29 (2018) 1456-1464. DOI:10.1016/j.cclet.2018.08.012 |
 2019, Vol. 30
2019, Vol. 30 

