b The First Clinical Medical College, Nanjing Medical University, Nanjing 210000, China
The growing interest in the application of nanomaterials to the biomedical field is largely due to its unique attractiveness to drug delivery [1, 2], diagnostics and imaging [3-5], synthetic vaccine development [6, 7], as well as the therapeutic properties of some nanomaterials themselves [8]. Although numerous kinds of nanomaterials have been developed for the biomedical application, only a very small number of them were translated into clinical studies. This is mainly caused by the consideration of biosafety.
Compared with the synthetic biomaterials, the use of natural biomaterials extracted from organisms for medical applications has attracted much more interest from scientists working in many fields [9]. For example, natural polyphenols, including (-)-epigallocatechin gallate (EGCG), and tannic acid, a major component of green tea, can be used for anti-cancer, anti-microbial, anti-virus and so on [10-12].
Melanins are a class of polyphenol compounds whose monomeric unit is an indole ring [13]. They are responsible for most of the brown, black, and grey pigmentations in animals, plants, and microbes, as well as in the human skin, hair and eyes [14]. It has been found to exhibit multiple functions in biosystems, including the protection of humans and animals from ultraviolet injury, antibiotic function, thermoregulation, free radical quenching, and some nervous system involvement [15-18]. Here, we introduce the biomedical application of melanin, as well as melanin-like biopolymers, such as polydopamine (PDA) nanoparticles.
Melanin is generally insoluble in water and common organic solvents. It only dissolved in strongly alkaline aqueous solution, which largely limits the application of melanin in biological and medical fields. For melanin nanoparticles (MNP), nanoscale melanin granules are able to combine the excellent properties ofmelanin and nanoparticles. MNPs have following advantages: 1) It has strong absorption in the near-infrared region [19]. 2) The melanin has numerous functional groups such as = O, -OH, –NH, and -COOH. These functional groups make melanin an ideal choice for chelating to metal ions, especially Cu, Fe, Mn [13]. 3) Paramagnetic feature is showed in melanin, which means high signal showed in T1 weighed and low signal showed in T2 weighed. 4) It owns good biocompatibility and degradation ability [20, 21]. Inspired by these, melaninbased nanoparticles has been widely used in photoacoustic (PAI)/ nuclear magnetic resonance (MRI)/positron emission tomography (PET) multi-mode imaging, photothermal therapy (PTT), chemotherapy and other carriers in recent years (some typical examples of such systems are summarized in Table 1).
|
|
Table 1 Representative melanin-based nanoprobes for in vivo imaging. |
2. Preparation 2.1. Melanin-like nanoparticles (bottom-up approach)
Melanin-like nanoparticles are widely studied nowadays. It is a major component of melanin, possesses excellent biocompatibility and is biodegradable in the body [22-24]. They are similar in structure and properties compared to eumelanin and have attracted considerable interest for various types of biological applications. The formation of these nanoparticles occurs by the oxidative polymerization of dopamine through a mechanism that resembles the biological pathway of melanin synthesis. Since mussel-inspired polydopamine emerged, it attracted more and more attention to be a wonderful biomaterial for surface coating and tissue engineering [25-27]. The polydopamine nanoparticles were also developed. For example, Liu et al. [19], Wang et al. [28], Wang et al. [29] and Pyo et al. [30] synthesized the melanin-like nanoparticles by the oxidation and self-polymerization of dopamine in a mixture containing water, ethanol, alkaline solution or oxidants (such as ammonia, KMNO4, H2O2) at room temperature. The average diameters of these three melanin-like nanoparticles were around 100 nm. Their size could be easily controlled by tuning the molar ratio of ammonia to dopamine. The main synthetic processes were shown in Fig. 1. In Ju et al.'s research [31], melanin-like nanoparticles were synthesized by neutralization of dopamine hydrochloride with NaOH in water, followed by autooxidation in the air, with an average diameter of 84 ±16 nm. Wu et al. [32] prepared melanin-like nanofilms, which are different from melanin-like nanoparticles. They were formed at an air/solution interface during the development of dopamine-melanin aggregates in a dopamine solution under static conditions, with granule sizes that varied from 500 nm to 1500 nm.

|
Download:
|
| Fig. 1. (a) Chemical synthesis route of melanin-like nanoparticles from dopamine; (b) TEM image of melanin-like nanoparticles. Scale bar, 1 μm. Reproduced with permission [19]. Copyright 2013, Wiley. | |
2.2. Nature melanin nanoparticles (top-down approach)
Some natural melanin exists in spherical particles, which is smaller in size. For this kind ofmelanin, melanin nanoparticles could be separated with centrifuging (18, 000 rpm, 15 min). For example, Wang et al. succeeded in getting MNPs from the cuttlefish's ink bag, with diameters of approximately 150–200 nm [33]. However, most of the natural melanin has a large size, irregular shape, insoluble in many liquid media. Here, this kind of melanin granules was first dispersed into water-soluble nanoparticles by Fan et al. [34]. To change the intrinsic poor water solubility of melanin, pristine melanin granule was first dissolved in a 0.1 mol/L NaOH and then neutralized under the assistance of sonication to decrease interchain aggregation. Ultrasmall MNP in high water monodispersity and homogeneity with a size of 4.5 ± 0.5 nm, MNP exhibited excellent water-solubility of 40 mg/mL and stability.
Furthermore, water-soluble MNP can be stored as a lyophilized powder for over six months and effectively re-dissolved in water allowinglong-termusage.The molecular weight of thisultrasmall-MNP is about 40kDa. The process of the preparation can be seen in Fig. 2.

|
Download:
|
| Fig. 2. (a) The preparation of water-soluble MNP; (b) TEM images of water-soluble MNP and MNP-PEG. Scale bar, 50 nm. Reproduced with permission [34]. Copyright 2014, American Chemical Society. | |
3. Molecular imaging 3.1. MR
Magnetic resonance imaging (MRI) is a frequently used imaging modality that has become increasingly important in the diagnosis of human disease [35]. It is noninvasive and versatile, without ionizing radiation, and can be acquired at high resolution for obtaining anatomical and functional information on soft tissues [36]. However, the low sensitivity inherent to MRI has led to the development of MRI contrast agents that increase sensitivity by catalytically shortening the transverse (T1) and longitudinal (T2) relaxation times of water protons [37, 38].
Gadolinium chelates (such as Gd-DOTA), which are widely used T1 contrast agent in clinical applications, have shortcomings such as fast metabolism in vivo; non-specific distribution in vivo; difficult to modify. Moreover, the ideal T1 contrast free metal ions (such as Gd3+ and Fe3+ show weak in vivo stability and cause severe toxicity. For example, it has been reported that the administration of Gd-based contrast agents to patients with renal dysfunction may induce the severe disease, nephrogenic systemic fibrosis [39, 40]. Therefore metal ions need to bound with or encapsulated into a carrier.
Since MNP shows intrinsic chelating ability with metal ions, it is considered that using MNP as a carrier chelates with Fe3+ or Gd3+ for MR imaging. Ju et al. introduced a highly efficient and optimized T1 MRI contrast agent by using synthetic MNPs, which provided not only a new platform with promising applications in diagnostic radiology and nanoprobe imaging but also an insight into the development of highly efficient and biocompatible melanin-based T1 MRI contrast agents [41]. Mn2+ was also chelated in MNPs which can be served as MRI agents [42]. The synthesis procedure and both T1 and T2 weighted imaging pictures are shown in Fig. 3.

|
Download:
|
| Fig. 3. (a) Synthesis procedure of MnEMNPs. The DL-DOPA precursor selfpolymerizes into MnEMNPs via a one-pot intrapolymerization doping strategy. The KMnO4 serves as the Mn source and an oxidant concurrently. (b) In vivo T1 weighted imaging and T2 weighted imaging of U87MG tumor-bearing mouse prior to and at different time points post-injection of PMnEMNPs. Reproduced with permission [42]. Copyright 2018, Wiley. | |
Moreover, in order to make nanoparticles access to targeted tissues, apart from enhanced permeability and retention (EPR) effect, attaching a targeting moiety is usually necessary. Fan et al. [34] used Fe-RGD-PEG-MNP as an MR imaging agent for U87MG tumor-bearing mice. With the increase of NP concentration, MR signal was significantly enhanced, suggesting Fe-RGD-PEG-MNP generate a high magnetic field gradient on their surface. The ratio of Fe3+ to PEG-MNP was nearly 90:1. Relaxivity values (r1; the slopes of each plot, L μmol-1 s-1) were 1.2 L μmol-1 s-1, demonstrating that MNP can be used as a platform for MRI. Yang et al. [43] embedded ultrasmall MNPs into the cavity of apoferritin (APF), which possesses targeting ability to transferrin receptor 1 (TfR1), overexpressed in numerous type of cancer cells [44]. The ratio of Fe3+ to MNP was nearly 1000:1, which was extremely high. Relativity values were determined to be 2.54 L μmol-1 s-1. This value is over 2 fold compared to the r1 value of Fan's work.
3.2. PETPET studies play an important role in the early detection of tumor metastasis [45], Alzheimer's and Parkinson's diseases [46], and cardiovascular disease [47]. It is the most mature molecular imaging technology at present, with the advantages of high sensitivity and is easy to quantify.
Nowadays, there is currently great interest in the application of nanoparticles for PET imaging [48-50]. This has led to the development of both organic and inorganic nanoparticles that have been functionalized so that radionuclides, targeting ligands, and polyethylene glycol can be attached to provide the imaging signal, target the particle, and alter the pharmacokinetics of the particle [51]. For example, Simone et al. used endothelial targeting of polymeric nanoparticles stably labeled with the PET imaging radioisotope iodine-124 [52]; Yang et al. utilized cRGD-functionalized, DOX-conjugated, and 64Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging [53]; Charles et al. reported the development and in vivo evaluation of a novel dual-PET/MR nanoparticle composed of a monocrystalline superparamagnetic iron oxide (SPIO) core and a physically adsorbed layer of PEGylated lipids [54].
As mentioned above, a number of positron emitting metal isotopes have begun to find widespread use in research and are moving towards medical applications recently [55]. Just like MRI, we can utilize the intrinsic chelating ability with metal ions of MNP, forming coordination bonds between these nuclides and MNP. Hong et al. prepared 64Cu-MNP, with the radiolabeling yield of 85%. The 64Cu-MNP displayed excellent stability in PBS solution [56]. After intravenous injection of 64Cu-MNP, in vivo study of PET of 64Cu- MNP showed high radioactive concentration in mouse liver. The result showed that MNP can be used as a platform for PET imaging.
To monitor the MNP-sorafenib (SRF) drug delivery system, Zhang et al. labeled the 64Cu to the MNP-SRF, with the labeling yield of 64% for micro-PET imaging [57]. At 4 h post-injection, the 64Cu radioactivity in the tumors increased in the PET images, compared to 2 h post-injection, and then decreased over time. The quantitative analysis showed the highest tumor 64Cu2+ uptake (5.5 ± 0.3% ID/g) at 4 h post-injection, and then a little-decreased uptake at 24 h (4.8 ± 0.4% ID/g), which showed that SRF transport to tumors using PEG-MNPs as carriers were flexible. Zhang et al. used 89Zr-MNP for pharmacokinetics study in iron-overloaded mice, with the labeling yield of 89.6 ±1.9% [58]. The result displayed higher liver uptakes, which were 14.02 ± 0.81, 13.39 ± 0.99, 12.71 ±1.02, and 11.64 ± 0.73% ID/g at 2, 4, 24 and 48 h p.i., respectively.
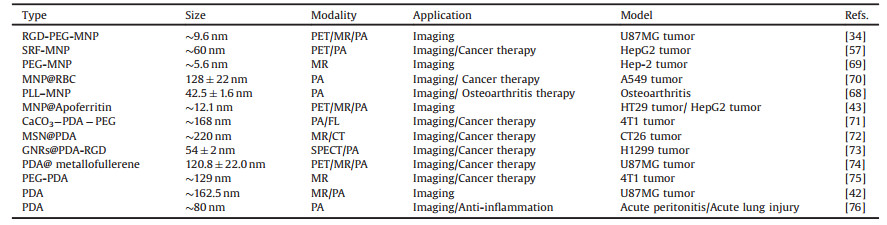
To further investigate the possible targeting property of MNPs, the 64Cu radiolabeled RGD-PEG-MNP were prepared in Fan's research [34]. The radiolabeling yield was about 80%. The PET imaging of 64Cu-RGD-PEG-MNP in U87 tumor-bearing mice revealed that the tumor uptake values increased with time to 24 h, and they were 4.75 ± 0.63, 5.87 ± 0.87, and 5.93 ± 0.89% ID/g at 2, 4, and 24 h, respectively. Then, similarly, to explore the targeting property of MNPs, Yang et al. [43], used 64Cu-APF-MNP in human colon cancer HT29 for PET imaging, which overexpresses TfR1. Different from Hong, Fan and Zhang, who used the chelating ability of MNP to label the 64Cu, Yang embedded ultrasmall MNPs into the cavity of APF. Then, 64Cu was loaded directly into AMF at pH 6 through multichannel. The labeling yield was about 80%. PET imaging results showed the tumor uptakes were 6.14 ± 0.77, 7.34 ± 0.93 and 6.74 ± 0.51% ID/g at 2, 4, and 24 h p.i., respectively, which is higher than that in Fan's research. The labeling process and PET images can be seen in Fig. 4. Altogether, these results demonstrated that MNP can be effectively acted as a PET imaging For its EPR effects, the higher uptakes and stability can be realized.
|
Download:
|
| Fig. 4. (a) Preparation of apoferrtin-encapsulated 64Cu labeled melanin (AMFMNP-64Cu); (b) Representative coronal small animal PET images of HT29 (top) and HepG2 (bottom) tumor-bearing mice at 1, 2, 4, 18 and 26 h after administration of AMF-MNP-64Cu; White circles indicate tumors location. Reproduced with permission [43]. Copyright 2015, Elsevier. carrier. | |
3.3. Photoacoustic (PA)
PA imaging is promising in the field of molecular imaging. PA imaging provides images of endogenous biological molecules such as melanin, microcalcification, and hemoglobin [59]. This imaging technique is based on the conversion of irradiated light energy, absorbed by target molecules, into thermal energy [60]. The target molecules generate acoustic waves (i.e., PA waves) because of transient thermoelastic expansion. Generally, this expansion occurs when the incident laser pulses are shorter than the diffusion time of the thermal energy induced in the molecules. An ultrasound transducer can detect the PA waves to reconstruct images. The optical absorbance of a target molecule determines the strength of the PA waves because the amount of thermal energy is related to the light energy absorbed by the molecule [61]. Few endogenous chromophores, such as hemoglobin, exist in the body as a target molecule for PA imaging. Therefore, the development of exogenous nanoprobes is necessary to expand the potential clinical application of PA imaging. Plasmonic noble metal nanoparticles [62], carbon-based nanomaterials [63] and other inorganic nanoparticles [64] were widely investigated as exogenous contrast agents for PA imaging in animal experiments because they are tunable for a high optical absorbance at a desired light wavelength. However, it remains unclear whether these types of inherently non-biological materials are safe for clinical use. In this respect, a biocompatible contrast agent for PA imaging based on organic materials is preferable and necessary [65].
Melanin molecules have much stronger optical absorption than surrounding skin in the near-infrared region. For its excellent biocompatibility, it is of great importance to use in the PA image.
To investigate the possibility of MNPs to be used as a photoacoustic agent, Fan et al. first studied the detection sensitivity of PEG-MNP in aqueous solution at increasing concentrations from 0.625 μmol/L to 20 μmol/L [34]. The PEG-MNP with 0.625 μmol/L was detected, and the photoacoustic signals increased linearly with the increase of PEG-MNP concentrations (R2 = 0.995). They then further investigated the in vivo PAI properties of RGD-PEGMNP. The mice showed an obvious increase of photoacoustic signal in tumors after injection with RGD-PEG-MNP at 4 h. The increased photoacoustic signal of RGD-PEG-MNP could be attributed to the enhanced permeability and retention (EPR) effect and the tumor targeting ability of RGD-PEG-MNP to αvβ3 integrin. Similarly, Yang et al. used MNP-APF as a carrier for PAI imaging. The tumor uptake reached 1.03 ± 0.02, 7.31 ± 2.33 and 24.15 ± 2.86 A U. at 0, 2 and 4 h p.i. [43].
Ju et al. [66] presented pH-responsive melanin-like nanoparticles, which aggregate in mildly acidic conditions. He also demonstrated that the physical aggregation of melanin-like nanoparticles result in an increase in the PA signal strength in the near-infrared window of biological tissue (i.e., 700 nm) without their absorption tuning. The PA signal strength of the bare melanin-like nanoparticles was 8 times lower than gold nanorods at a given weight concentration. After physical aggregation of melanin-like nanoparticles, the PA signals were similar to gold nanorods, suggesting melanin-like nanoparticles exhibit potential in PA imaging. Using tubing phantoms, Lipo et al. showed that optoacoustic efficiency of MNPs is similar to that of gold nanoparticles, which is a widely concerned optoacoustic agent [67]. All these results showed that MNP was an excellent nanoprobe for PAI.
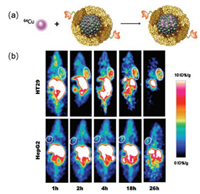
Chen et al. constructed poly-L-lysine encapsulated MNPs which have positive surface charge (+32.5 ± 9.3 mV). These PLL-MNPs with high PA intensity, photostability and biocompatibility exhibited about a two-fold stronger PA signal in a normal joint than an osteoarthritis joint. The in vivo PA images of PLL-MNPs in joints were shown in Fig. 5.
|
Download:
|
| Fig. 5. (a) Schematic diagram of PAI mediated by MNP. (b) PA images of knee joints after the intra-articular administration of PLL–MNPs for 3 h. The representative PA maximum intensity projection (MIP) images with the PA image for PLL–MNPs. It showed decreased accumulation of contrast agents in the OA joint (white circle). Reproduced with permission [68]. Copyright 2018, the Royal Society of Chemistry. | |
4. Disease treatment 4.1. Drug delivery
Many hydrophobic drugs should be administered orally, therefore easily leading to side effects [77] and low absorption rates [78]. Nowadays, nanotechnology for drug delivery can improve treatment efficiency and reduce side effects, which has received great attention [79]. A broad range of nanocarriers, to date, with diverse sizes, architectures, and surface properties have been designed [80-83]. For inorganic nanosystems, such as quantum dots [84], metal [85, 86], and metal oxide frameworks [87], their potential long-term toxicity could limit clinical translation. Organic nanosystems, including liposomes [88], polymer nanoparticles (NPs) [89], micelles [90], and dendrimers [91] have also been developed for drug delivery. Moreover, using endogenous organic nanostructures, such as cells [92] and ferritines [93], appears more advantages due to their native biocompatibility and biodegradability. Obviously, MNP, as a naturally occurring pigment, belongs to the endogenous organic nanostructures.
Previously, scientists had observed the strong binding capability of melanin to drugs and attributed such drug–melanin conjugates to the π–π interaction between the aromatic rings on drug and melanin (having dihydroxyindole/indolequinone segments) [58, 94].
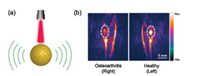
Considering this, Zhang constructed sorafenib (SRF, a multikinase inhibitor for treating unresectable hepatocellular carcinoma [95])-MNP–drug system through their π–π interaction, which improved the absorption rates and effectiveness of chemotherapy (Fig. 6) [57]. The volume of HepG2 tumors treated with SRF-MNPs by tail-vein injection was only 679 mm3, which showed the higher anti-tumor effect as compared with 1105 mm3 of the oral SRF group (P < 0.05). Wang et al. [28] reported a polydopamine (PDA) nanoparticle-knotted poly(ethylene glycol) (PEG) hydrogel for ondemand drug delivery. Anticancer drugs 7-ethyl-10-hydroxycamptothecin (SN38) loaded on PDA nanoparticles via π–π interaction in the gel exhibited minimal leakage at physiological conditions and could be released in an on-demand fashion upon near-infrared light exposure. In PDANP - SN38, group, the tumor volume was nearly 3 times smaller than the PBS group. Still through π–π interaction, Wang et al. reported a PDA-DOX/SN38 drug delivery system for cancer treatment [29]. They compared the photothermal therapy with chemotherapy (PT/CT). The tumor growth in the PT/CT group was completed suppressed. While in the PBS group, the tumor volume was more than 5 times of the PT/CT group.
|
Download:
|
| Fig. 6. (a) The preparation of sorafenib-loaded melanin (SRF-MNP); (b) Representative photos of HepG2 tumor-bearing mice before and day 20 after tail-vein-injected treatment with PBS, PEG-MNP, SRF-MNP, and oral treatment with SRF. The white arrows refer to the tumor position; (c) Tumor growth curves of HepG2 tumor-bearing mice after various treatments. Reproduced with permission [57]. Copyright 2015, Wiley. | |
Using supercritical fluid technology, a clean route for impregnation, Araújo et al. [96] showed that MNPs could be straightforwardly impregnated with metronidazole(MZ), chosen as a model antibiotic drug, taking advantage of the high diffusivity, low viscosity and high density of supercritical CO2, leaving no residues in the final product. The release plateau at physiologic pH 7.4 was obtained in about 9 h, corresponding to 87% of MZ release. At pH 2.2, the amount of drug released does not exceed 10% for the same period of time. This release profile showed that melanin strongly responds to pH thus, having a significant control on drug release, which is a very interesting feature for the treatment of intestine and colon diseases, which would greatly benefit with pH-targeting.
These interesting results allied to its high biocompatibility can prompt the use of melanin as a novel biomaterial for the potential use in the pharmaceutical and biomedical fields.
4.2. Photothermal therapy (PTT)As a noninvasive cancer treatment, PTT has earned much attention in recent years due to its localized tumor ablation and minimal heating damage to adjacent normal tissues [70]. The mechanism of PTT is that photothermal agents strongly absorb and covert near infrared(NIR) light into thermal energy after their adequate accumulation within tumors [97]. Currently, various materials have been explored as effective photothermal agents, including inorganic nanomaterials (e.g., gold-, carbon-, palladium-, and magnetic nanoparticles) NIR dye (indocyanine green poly (ethylenedioxythiophene) (PEDOT)) and other nanomaterials (e.g., porphysomes, Prussian blue). Despite their extraordinary photothermal effect, the long-term safety of these synthetic nanomaterials in vivo may still limit their further clinical application in cancer treatment. Therefore, it will be of great clinical value to develop biocompatible and biodegradable photothermal nanomaterials with high photothermal efficiency for in vivo cancer PTT.
With the excellent biocompatibility and photothermal effect of melanin, whose absorption can extend to NIR regions, and melanin-like nanoparticles, it cannot be more suitable for the photothermal agents. Liu et al. synthesized melanin-like colloidal nanospheres as effective photothermal agents for in vivo cancer therapy and achieved as high as 40% photothermal conversion efficiency [19]. In another research of Cheng group, PC-9 tumorbearing mice in PTT groups were treated with NIR irradiation at a power density of 3.6 W/cm2 for 8 min at a series of time points after each injection [29]. The tumor growth in the PDA group was considerably suppressed, whose volume was 2 times smaller than the chemotherapy group, suggesting that PTT significantly contributed to tumor suppression.
Compared with those melanin-like nanoparticles described above, the natural melanin nanoparticles extracted from living organisms attracted more alluring attention for cancer PTT because of their native biocompatibility and biodegradability, which could effectively eliminate the side-effects as well as default metabolism in a biological system.
For example, Chu et al. extracted natural melanin nanoparticles from black sesame seeds. He demonstrated that melanin nanoparticles can be used for SLN mapping and cancer PTT [9]. The intratumoral injection of liposome-melanin nanocomposites significantly inhibited the growth of tumor cells in mice following PTT. Compared with blank-liposome, the tumor volume in liposome-melanin group decreased almost 250%.
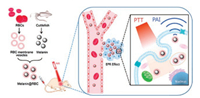
Natural melanin nanoparticles extracted from living cuttlefish were also explored as effective photothermal agents and with biomimetic technology [70]. Red blood cell (RBC) membrane camouflaged melanin (Melanin@RBC) nanoparticles were developed as a platform for in vivo antitumor PTT (Fig. 7). First, the photothermal conversion efficiency between melanin nanoparticles and melanin-like (PDA) nanoparticles were compared. The results showed that, with the similar 808 nm, 2 W/cm2 exposed, melanin nanoparticles exhibited significantly higher temperature increment (△T = 37.2 ℃) than PDA nanoparticles (△T = 24.5 ℃) at equivalent concentration, suggesting that natural melanin nanoparticles have better photothermal performance over melanin-like PDA nanoparticles. The photothermal conversion efficiency was 40% in melanin nanoparticles, which was high than melanin-like polydopamine nanoparticles, 29%, as well as favorable biocompatibility. Then he did the PTT in A549 tumorbearing mice. Upon irradiation by an 808-nm laser, the developed Melanin@RBC nanoparticles exhibited significantly 4 times higher PTT efficacy than that of bare melanin nanoparticles in A549 tumor-bearing mice. These results showed that the developed Melanin@RBC platform could have great potential in clinics for anticancer PTT.
|
Download:
|
| Fig. 7. Schematic illustration of red blood cell membrane camouflaged melanin nanoparticles for enhanced photothermal therapy. Reproduced with permission [70]. Copyright 2017, Elsevier. | |
4.3. Antioxidative therapy
Elevated levels of reactive oxygen and nitrogen species (RONS) are involved in the development and progression of a variety of diseases such as cancer, neurological disorders, and inflammatory diseases [98, 99]. In general, the scavenging of RONS has some help in the treatment of these diseases [100, 101]. Melanin, as an endogenous antioxidant of the organism, have the potent antioxidant capacity [102]. Even melanin-like nanoparticles exhibit strong free radical scavenging ability [31].
Ischemic strokes occur as a result of an obstruction within a blood vessel supplying blood to the brain which will lead to the brain injury [103]. Subsequently, the oxidative stress will increase in the brain. Melanin-like nanoparticles were developed as an antioxidative treatment to protect the brain damage in ischemic stroke [16]. PEGylated melanin-like nanoparticles exhibit broad antioxidative activities against multiple toxic RONS. Preinjection of PEGylated melanin-like nanoparticles can significantly decrease the infarct area of the ischemic brain in a rat model of ischemic stroke.
Prolonged exposure to ultraviolet (UV) radiation can have many potential adverse effects on human skin, such as sunburn, skin ageing and skin cancer [104]. The excessive RONS induced by exposure to UV radiation has damage to both DNA and mitochondria of cells. Melanin sunscreens, inspired by skin pigmentation, were developed to protect the skin from UV irradiation [105]. Melanin sunscreens with simple, rapid, and versatile fabrication approach showed improved photoprotective capacities both on cells and mice.
4.4. Iron overload therapyAs we known, iron overload would cause long-term repeated blood transfusions, increased intestinal iron absorption, or ineffective erythropoiesis or bone marrow hematopoietic activity, which results in the abnormal function of tissues and organs, even be life-threatening [106].
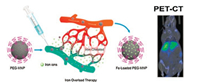
Yan et al., for the first time, reported simply using MNPs as an efficient endogenous nano-drug for iron overload therapy (Fig. 8) [107]. In comparison with traditional deferoxamine (DFO), the most commonly used reagent in clinical treatment, MNPs exhibited superior efficiency in iron excretion and a favorable safety profile. Furthermore, their tremendously longer circulating half-life, which is about 200-fold increased over DFO (17 h vs. 5 min), can efficiently reduce the frequency of administration and the total dosage (35–100 mg/kg vs. 750 mg/kg) for the iron overload treatment.
|
Download:
|
| Fig. 8. Schematic illustration of utilizing ultrasmall PEG-MNPs as metal-ion scavengers to excrete excess iron for overload therapy. Reproduced with permission [107]. Copyright 2016, the Royal Society of Chemistry. | |
5. Conclusions and outlook
In this review, we summarized the reliable method for the preparation of water-soluble MNPs which has laid down a foundation for its future biomedical applications. The MNPs have multiple binding sites that make it more rapid and stable when binding with metal ions and hydrophobic drugs. The abundant quinone on the surface of MNP makes it surface modification easily. Thus, it can be easily envisioned that MNP can serve as a nanoplatform not only for molecular imaging but also for disease treatment. The robust metal chelating made it a perfect MR and PET imaging nanoprobe. The inherent absorbance at NIR range makes it PA imaging probe. Therefore, MNP can also be developed as PTT agent. The strong radical scavenging ability also makes it an ideal antioxidant therapeutic. Although MNP has already developed plenty of biomedical applications, many challenges still need to be overcome for clinical translation in the future. For instance, the exact structure of natural melanin is still unsettled and the specific synthesis mechanism needs to be studied urgently. The large scale synthesis of water-soluble melanin or polydopamine with controllable and narrow size is also a great challenge. Furthermore, the long-term toxicity in vivo still remain to be systematically investigated. At the same time, the degradation and metabolism mechanism of MNPs in vivo is not fully understood. After all these issues to be settled, the clinical translation of MNPs may succeed and its applications in biomedicine can be further explored.
AcknowledgementsWe thank financial support from the National Natural Science Foundation of China (Nos. 31671035, 51473071, 51803082), Natural Science Foundation of Jiangsu Province (No. BK20170204) and Jiangsu Provincial Medical Innovation Team (No. CXTDA2017024), Innovation Capacity Development Plan of Jiangsu Province (No. BM2018023) and Jiangsu Provincial Key Medical Discipline (No. ZDXKA2016017).
| [1] |
P. Couvreur, Adv. Drug Deliv. Rev. 65 (2013) 21-23. DOI:10.1016/j.addr.2012.04.010 |
| [2] |
E. Blanco, H. Shen, M. Ferrari, Nat. Biotechnol 33 (2015) 941. DOI:10.1038/nbt.3330 |
| [3] |
S.Y. Lee, S.I. Jeon, S. Jung, I.J. Chung, C.H. Ahn, Adv. Drug Deliv. Rev. 76 (2014) 60-78. DOI:10.1016/j.addr.2014.07.009 |
| [4] |
I.A. Aljuffali, C.L. Fang, C.H. Chen, J.Y. Fang, Curr. Pharm. Des. 22 (2016) 4219-4231. DOI:10.2174/1381612822666160620072539 |
| [5] |
S.M. Park, A. Aalipour, O. Vermesh, J.H. Yu, S.S. Gambhir, Nat. Rev. Mater 2 (2017) 17014. DOI:10.1038/natrevmats.2017.14 |
| [6] |
M.A. Swartz, S. Hirosue, J.A. Hubbell, Sci. Transl. Med 4 (2012) 148rv9. |
| [7] |
D.J. Irvine, M.C. Hanson, K. Rakhra, T. Tokatlian, Chem. Rev. 115 (2015) 11109-11146. DOI:10.1021/acs.chemrev.5b00109 |
| [8] |
P.P. Adiseshaiah, R.M. Crist, S.S. Hook, S.E. McNeil, Nat. Rev. Clin. Oncol. 13 (2016) 750-765. DOI:10.1038/nrclinonc.2016.119 |
| [9] |
M. Chu, W. Hai, Z. Zhang, et al., Biomaterials 91 (2016) 182-199. DOI:10.1016/j.biomaterials.2016.03.018 |
| [10] |
J.E. Chung, S. Tan, S.J. Gao, et al., Nat. Nanotechnol. 9 (2014) 907-912. DOI:10.1038/nnano.2014.208 |
| [11] |
Y. Cao, R. Cao, Nature 398 (1999) 381-381. DOI:10.1038/18793 |
| [12] |
S. Garbisa, S. Biggin, N. Cavallarin, et al., Nat. Med. 5 (1999) 1216-1216. DOI:10.1038/15145 |
| [13] |
H. Thaira, K. Raval, V. Manirethan, R.M Balakrishnan, Sep. Sci. Technol (2018) 1-10. |
| [14] |
K.P. Watts, R.G. Fairchild, D.N. Slatkin, et al., Cancer Res. 41 (1981) 467-472. |
| [15] |
L. Jacquin, C. Récapet, P. Bouche, G. Leboucher, J. Gasparini, Behav. Ecol. 23 (2012) 907-915. DOI:10.1093/beheco/ars055 |
| [16] |
Y. Liu, K. Ai, X. Ji, et al., J. Am. Chem. Soc. 139 (2017) 856-862. DOI:10.1021/jacs.6b11013 |
| [17] |
J.D. Simon, Acc. Chem. Res. 33 (2000) 307-313. DOI:10.1021/ar970250t |
| [18] |
A. Topete, M. Alatorre-Meda, P. Iglesias, et al., ACS Nano 8 (2014) 2725-2738. DOI:10.1021/nn406425h |
| [19] |
Y. Liu, K. Ai, J. Liu, et al., Adv. Mater. 25 (2013) 1353-1359. DOI:10.1002/adma.v25.9 |
| [20] |
J. Lin, M. Wang, H. Hu, et al., Adv. Mater. 28 (2016) 3273-3279. DOI:10.1002/adma.201505700 |
| [21] |
C.J. Bettinger, J.P. Bruggeman, A. Misra, J.T. Borenstein, R. Langer, Biomaterials 30 (2009) 3050-3057. DOI:10.1016/j.biomaterials.2009.02.018 |
| [22] |
Y. Liu, K. Ai, L. Lu, Chem. Rev. 114 (2014) 5057-5115. DOI:10.1021/cr400407a |
| [23] |
M.E. Lynge, R. van der Westen, A. Postma, B. Städler, Nanoscale 3 (2011) 4916-4928. DOI:10.1039/c1nr10969c |
| [24] |
X. Liu, J. Cao, H. Li, et al., ACS Nano 7 (2013) 9384-9395. DOI:10.1021/nn404117j |
| [25] |
H. Lee, S.M. Dellatore, W.M. Miller, P.B. Messersmith, Science 318 (2007) 426-430. DOI:10.1126/science.1147241 |
| [26] |
S.M. Kang, I. You, W.K. Cho, et al., Angew. Chem. Int. Ed. 49 (2010) 9401-9404. DOI:10.1002/anie.201004693 |
| [27] |
W.B. Tsai, W.T. Chen, H.W. Chien, W.H. Kuo, M.J. Wang, Acta Biomater. 7 (2011) 4187-4194. DOI:10.1016/j.actbio.2011.07.024 |
| [28] |
X. Wang, C. Wang, X. Wang, et al., Chem. Mater. 29 (2017) 1370-1376. DOI:10.1021/acs.chemmater.6b05192 |
| [29] |
X. Wang, J. Zhang, Y. Wang, et al., Biomaterials 81 (2016) 114-124. DOI:10.1016/j.biomaterials.2015.11.037 |
| [30] |
J. Pyo, K.Y. Ju, J.K. Lee, J. Photoch, Photobiol. B 160 (2016) 330-335. DOI:10.1016/j.jphotobiol.2016.04.022 |
| [31] |
K.Y. Ju, Y. Lee, S. Lee, S.B. Park, J.K. Lee, Biomacromolecules 12 (2011) 625-632. DOI:10.1021/bm101281b |
| [32] |
T.F. Wu, J.D. Hong, Biomacromolecules 16 (2015) 660-666. |
| [33] |
Y. Wang, T. Li, X. Wang, et al., Biomacromolecules 17 (2016) 3782-3789. DOI:10.1021/acs.biomac.6b01298 |
| [34] |
Q. Fan, K. Cheng, X. Hu, et al., J. Am. Chem. Soc. 136 (2014) 15185-15194. DOI:10.1021/ja505412p |
| [35] |
M.D. Fox, M.E. Raichle, Nat. Rev. Neurosci. 8 (2007) 700-711. DOI:10.1038/nrn2201 |
| [36] |
E. Terreno, D.D. Castelli, A. Viale, S. Aime, Chem. Rev. 110 (2010) 3019-3042. DOI:10.1021/cr100025t |
| [37] |
Y. Li, Y. Huang, Z. Wang, et al., Small 12 (2016) 668-677. DOI:10.1002/smll.201502754 |
| [38] |
A. Rajaee, X. Wensheng, L. Zhao, et al., J. Biomed. Nanotechnol. 14 (2018) 1159-1168. DOI:10.1166/jbn.2018.2553 |
| [39] |
S. Aime, P. Caravan, J. Magn. Reson. Imaging 30 (2009) 1259-1267. DOI:10.1002/jmri.v30:6 |
| [40] |
A. Kribben, O. Witzke, U. Hillen, et al., J. Am. Coll. Cardiol. 53 (2009) 1621-1628. DOI:10.1016/j.jacc.2008.12.061 |
| [41] |
K.Y. Ju, J.W. Lee, G.H. Im, et al., Biomacromolecules 14 (2013) 3491-3497. DOI:10.1021/bm4008138 |
| [42] |
H. Liu, C. Chu, Y. Liu, et al., Adv. Sci 5 (2018) 1800032. DOI:10.1002/advs.v5.7 |
| [43] |
M. Yang, Q. Fan, R. Zhang, et al., Biomaterials 69 (2015) 30-37. DOI:10.1016/j.biomaterials.2015.08.001 |
| [44] |
P. Huang, P. Rong, A. Jin, et al., Adv. Mater. 26 (2014) 6401-6408. DOI:10.1002/adma.201400914 |
| [45] |
K. Ozturk, R. Gawande, M. Gencturk, et al., Clin. Imaging 51 (2018) 217-228. DOI:10.1016/j.clinimag.2018.05.018 |
| [46] |
I. Sarikaya, Nucl. Med. Commun. 36 (2015) 775-781. DOI:10.1097/MNM.0000000000000320 |
| [47] |
V.R. Pell, F. Baark, F. Mota, J.E. Clark, R. Southworth, Curr. Cardiovasc. Imaging Rep 11 (2018) 7. DOI:10.1007/s12410-018-9447-3 |
| [48] |
E. Phillips, O. Penate-Medina, P.B. Zanzonico, et al., Sci. Transl. Med 6 (2014) 149. |
| [49] |
X. Sun, W. Cai, X. Chen, Acc. Chem. Res. 48 (2015) 286-294. DOI:10.1021/ar500362y |
| [50] |
Y. Zhan, E.B. Ehlerding, S. Shi, et al., J. Biomed. Nanotechnol. 14 (2018) 900-909. DOI:10.1166/jbn.2018.2498 |
| [51] |
M.J. Welch, C.J. Hawker, K.L. Wooley, J. Nucl. Med. 50 (2009) 1743-1746. DOI:10.2967/jnumed.109.061846 |
| [52] |
E.A. Simone, B.J. Zern, A.M. Chacko, et al., Biomaterials 33 (2012) 5406-5413. DOI:10.1016/j.biomaterials.2012.04.036 |
| [53] |
X. Yang, H. Hong, J.J. Grailer, et al., Biomaterials 32 (2011) 4151-4160. DOI:10.1016/j.biomaterials.2011.02.006 |
| [54] |
C. Glaus, R. Rossin, M.J. Welch, G. Bao, Bioconjug. Chem. 21 (2010) 715-722. DOI:10.1021/bc900511j |
| [55] |
T.W. Price, J. Greenman, G.J. Stasiuk, Dalton Trans. 45 (2016) 15702-15724. DOI:10.1039/C5DT04706D |
| [56] |
S.H. Hong, Y. Sun, C. Tang, et al., Bioconjug. Chem. 28 (2017) 1925-1930. DOI:10.1021/acs.bioconjchem.7b00245 |
| [57] |
R. Zhang, Q. Fan, M. Yang, et al., Adv. Mater. 27 (2015) 5063-5069. DOI:10.1002/adma.201502201 |
| [58] |
P. Zhang, Y. Yue, D. Pan, et al., Nucl. Med. Biol. 43 (2016) 529-533. DOI:10.1016/j.nucmedbio.2016.05.014 |
| [59] |
J. Kang, E.K. Kim, G.R. Kim, et al., J. Biophotonics 8 (2015) 71-80. DOI:10.1002/jbio.201300100 |
| [60] |
G.J. Diebold, A.C. Beveridge, T.J. Hamilton, J. Acoust. Soc. Am. 112 (2002) 1780-1786. DOI:10.1121/1.1508788 |
| [61] |
J. Kang, E.K. Kim, J. Young Kwak, et al., Appl. Phys. Lett 99 (2011) 153702. DOI:10.1063/1.3651333 |
| [62] |
J.W. Kim, E.I. Galanzha, E.V. Shashkov, H.M. Moon, V.P. Zharov, Nat. Nanotechnol. 4 (2009) 688-694. DOI:10.1038/nnano.2009.231 |
| [63] |
D. Pan, X. Cai, C. Yalaz, et al., ACS Nano 6 (2012) 1260-1267. DOI:10.1021/nn203895n |
| [64] |
G. Ku, M. Zhou, S. Song, et al., ACS Nano 6 (2012) 7489-7496. DOI:10.1021/nn302782y |
| [65] |
J.F. Lovell, C.S. Jin, E. Huynh, et al., Nat. Mater. 10 (2011) 324-332. DOI:10.1038/nmat2986 |
| [66] |
K.Y. Ju, J. Kang, J. Pyo, et al., Nanoscale 8 (2016) 14448-14456. DOI:10.1039/C6NR02294D |
| [67] |
A. Liopo, R. Su, A.A. Oraevsky, Photoacoustics 3 (2015) 35-43. DOI:10.1016/j.pacs.2015.02.001 |
| [68] |
L. Chen, Y. Ji, X. Hu, et al., Nanoscale 10 (2018) 13471-13484. DOI:10.1039/C8NR03791D |
| [69] |
W. Xu, J. Sun, L. Li, et al., Biomater. Sci. 6 (2017) 207-215. |
| [70] |
Q. Jiang, Z. Luo, Y. Men, et al., Biomaterials 143 (2017) 29-45. DOI:10.1016/j.biomaterials.2017.07.027 |
| [71] |
Z. Dong, L. Feng, Y. Hao, et al., J. Am. Chem. Soc. 140 (2018) 2165-2178. DOI:10.1021/jacs.7b11036 |
| [72] |
X. Ding, X. Hao, D. Fu, et al., Nano Res. 10 (2017) 3124-3135. DOI:10.1007/s12274-017-1530-6 |
| [73] |
L. Zhang, H. Su, J. Cai, et al., ACS Nano 10 (2016) 10404-10417. DOI:10.1021/acsnano.6b06267 |
| [74] |
S. Wang, J. Lin, Z. Wang, et al., Adv. Mate 29 (2017) 1701013. DOI:10.1002/adma.201701013 |
| [75] |
Z. Dong, H. Gong, M. Gao, et al., Theranostics 6 (2016) 1031-1042. DOI:10.7150/thno.14431 |
| [76] |
H. Zhao, Z. Zeng, L. Liu, et al., Nanoscale 10 (2018) 6981-6991. DOI:10.1039/C8NR00838H |
| [77] |
J. Chen, A.Y. Sheu, W. Li, et al., J. Control. Release 184 (2014) 10-17. DOI:10.1016/j.jconrel.2014.04.008 |
| [78] |
G. Wang, J. Wang, W. Wu, et al., Expert Opin. Drug Deliv. 12 (2015) 1475-1499. DOI:10.1517/17425247.2015.1021681 |
| [79] |
W.I. Choi, J.Y. Kim, C. Kang, et al., ACS Nano 5 (2011) 1995-2003. DOI:10.1021/nn103047r |
| [80] |
H.L. Hauksdóttir, T.J. Webster, J. Biomed. Nanotechnol. 14 (2018) 510-525. DOI:10.1166/jbn.2018.2521 |
| [81] |
C. Yao, J. Tian, H. Wang, et al., Chin. Chem. Lett. 28 (2017) 893-899. DOI:10.1016/j.cclet.2017.01.005 |
| [82] |
W. Gao, Y. Hu, L. Xu, et al., Chin. Chem. Lett. 29 (2018) 1795-1798. DOI:10.1016/j.cclet.2018.05.022 |
| [83] |
K. Zhang, P.P. Yang, J.P. Zhang, L. Wang, H. Wang, Chin. Chem. Lett. 28 (2017) 1808-1816. DOI:10.1016/j.cclet.2017.07.001 |
| [84] |
C.M. Carpenter, C. Sun, G. Pratx, et al., Opt. Express 20 (2012) 11598-11604. DOI:10.1364/OE.20.011598 |
| [85] |
S. Jelveh, D.B. Chithrani, Cancers 3 (2011) 1081-1110. DOI:10.3390/cancers3011081 |
| [86] |
Y.P. Jia, B.Y. Ma, X.W. Wei, Z.Y. Qian, Chin. Chem. Lett. 28 (2017) 691-702. DOI:10.1016/j.cclet.2017.01.021 |
| [87] |
X. Cao, Z. Yin, H. Zhang, Energy Environ. Sci. 7 (2014) 1850-1865. DOI:10.1039/C4EE00050A |
| [88] |
L. Jiang, L. Li, X. He, et al., Biomaterials 52 (2015) 126-139. DOI:10.1016/j.biomaterials.2015.02.004 |
| [89] |
C.J. Cheng, G.T. Tietjen, J.K. Saucier-Sawyer, W.M. Saltzman, Nat. Rev. Drug Discov. 14 (2015) 239-247. DOI:10.1038/nrd4503 |
| [90] |
K. Kataoka, A. Harada, Y. Nagasaki, Adv. Drug Deliv. Rev. 47 (2001) 113-131. DOI:10.1016/S0169-409X(00)00124-1 |
| [91] |
P. Kesharwani, K. Jain, N.K. Jain, Prog. Polym. Sci. 39 (2014) 268-307. DOI:10.1016/j.progpolymsci.2013.07.005 |
| [92] |
K. Tang, Y. Zhang, H. Zhang, et al., Nat. Commun 3 (2012) 1282. DOI:10.1038/ncomms2282 |
| [93] |
D.J.E. Huard, K.M. Kane, F.A. Tezcan, Nat. Chem. Biol. 9 (2013) 169-176. DOI:10.1038/nchembio.1163 |
| [94] |
R.M. Ings, Drug Metab. Rev. 15 (1984) 1183-1212. DOI:10.3109/03602538409033561 |
| [95] |
J.M. Llovet, S. Ricci, V. Mazzaferro, et al., N. Engl. J. Med. 359 (2008) 378-390. DOI:10.1056/NEJMoa0708857 |
| [96] |
M. Araújo, R. Viveiros, T.R. Correia, et al., Int. J. Pharm. 469 (2014) 140-145. DOI:10.1016/j.ijpharm.2014.04.051 |
| [97] |
J.U. Menon, P. Jadeja, P. Tambe, et al., Theranostics 3 (2013) 152-166. DOI:10.7150/thno.5327 |
| [98] |
C. Nathan, A. Cunningham-Bussel, Nat. Rev. Immunol. 13 (2013) 349-361. DOI:10.1038/nri3423 |
| [99] |
K.J. Barnham, C.L. Masters, A.I. Bush, Nat. Rev. Drug Discov. 3 (2004) 205-214. DOI:10.1038/nrd1330 |
| [100] |
J.E. Slemmer, J.J. Shacka, M. Sweeney, J.T. Weber, Curr. Med. Chem. 15 (2008) 404-414. DOI:10.2174/092986708783497337 |
| [101] |
R. Bavarsad Shahripour, M.R. Harrigan, A.V. Alexandrov, Brain Behav. 4 (2014) 108-122. DOI:10.1002/brb3.2014.4.issue-2 |
| [102] |
E.S. Jacobson, S. Tinnell, J. Bacteriol. 175 (1993) 7102-7104. DOI:10.1128/jb.175.21.7102-7104.1993 |
| [103] |
J. Castillo, R. Leira, M.M. García, et al., Stroke 35 (2004) 520-526. DOI:10.1161/01.STR.0000109769.22917.B0 |
| [104] |
F.P. Noonan, J.A. Recio, H. Takayama, et al., Nature 413 (2001) 271-272. DOI:10.1038/35095108 |
| [105] |
C. Wang, D. Wang, T. Dai, et al., Adv. Funct. Mater 28 (2018) 1802127. DOI:10.1002/adfm.v28.33 |
| [106] |
A. Taher, Semin. Hematol 42 (2005) S5-S9. DOI:10.1053/j.seminhematol.2005.01.005 |
| [107] |
J. Yan, Y. Ji, P. Zhang, et al., J. Mater. Chem. B 4 (2016) 7233-7240. DOI:10.1039/C6TB01558A |
 2019, Vol. 30
2019, Vol. 30