b Research Institute for Science and Technology, Photocatalysis International Research Center, Tokyo University of Science, Chiba 278-8510, Japan;
c Henan Key Laboratory of Polyxometalate Chemistry, Institute of Molecular and Crystal Engineering, College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, China
Nowadays emission of carbon dioxide (CO2) and pollution of waters by organic wastes are considered to be worldwide environmental concerns [1]. Hence, the development of technologies for CO2 and wastewater treatment is of high significance in environmental protection. Moreover, the method of simultaneous CO2 reduction and wastewater purification seems to be an ideal strategy to address problems of today's gradually-depleted energy resources over the globe [2]. In the recent technologies, electrochemical methods have attracted great interest because their principal reagent is only electrons, which are inherently clean species in chemical reactions [3]. Electrochemical reduction of CO2 can produce a variety of organic compounds which can be used as feedstock forchemical conversion into hydrocarbon fuels [4-8]. This process is of interest for the recycling of CO2 as an energy carrier, thereby reducing its accumulation in the atmosphere. It is known that direct electron transfer to CO2 molecules require a high overpotential, which means the competitive process from hydrogen evolution should be overcome.
Boron-doped diamond (BDD) electrodes are considered as ideal candidate for both CO2 conversion and wastewater disinfection, because BDD possesses superior chemical and dimensional stability, high resistance to corrosion, low background currents, and a wide potential window [3, 9]. In comparison with many other electrode materials (such as glass carbon, platinum, gold), the wide potential window of BDD can suppress the competition of hydrogen evolution with CO2 reaction. Past research has found when using as an anode, BDD has the ability to transform CO2 to formaldehyde with a high yield through a 4-electron reduction process [10]. This finding has important significance since formaldehyde is of high industrial value.
On the other hand, BDD with environmental compatibility provides versatile and energy efficiency for the degradation of hazard organics in wastewaters [11]. Compared with traditional anodes, BDD has a high overpotential for oxygen evolution as well as for hydrogen evolution, which allows the generation of active hydroxyl radical (OH·) by water splitting (Reaction (1)). The generation of OH· at high concentration can react with organics diffusing to the BDD surface, mainly transforming into CO2 and H2O.

|
(1) |
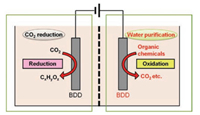
Based on these distinct advantages of BDD, in this research we design a dual-functional electrochemical device using two BDD electrodes as both the anode and the cathode, which can simultaneously perform CO2 reduction and wastewater purification in one redox process. Potassium hydrogen phthalate (KHP) was employed to test the ability for wastewater treatment and to estimate the influence on the CO2 reduction at the cathodic side. This novel system appears to be feasible for the use in the reduction of CO2 during the degradation in wastewater treatment, thus aiding the sustainable development for energy and environment.
KHP (1 mmol/L) and Na2SO4 (0.1 mol/L) (Analytical grade, Nacalai Tesque, Japan) was used as received. BDD (with microcrystalline surface) thin films were prepared by a microwave plasma assisted chemical vapor deposition (MPCVD) system (ASTeX Corp. Woburn, MA). The electrochemical apparatus was indicated in Fig. 1. The electrochemical treatment was carried out in a two-compartment cell (each 50 mL), with an Ag/AgCl electrode as the reference. Two BDD electrodes, with the same areas 5.5 cm2, were used as the working electrode and the counter electrode respectively. 0.1 mol/L Na2SO4 aqueous solution was prepared as the electrolyte. A Nafion ion exchange membrane (Aldrich) was used as the diaphragm. Firstly, N2 was bubbled into the electrolyte for 30 min at a rate of 100 mL/min, followed by CO2 bubbling for 90 min. Then the obtained CO2-saturated electrolyte was electrolytically reduced at a cathodic potential for 60 min. All cyclic voltammograms (CVs) were taken at a scan rate of 100 mV/s by magnetic stirring at atmospheric conditions. The soluble products in the electrolyte were analyzed by HPLC method. The Faradaic efficiency of formaldehyde was calculated by the production amount per assumed charge energy.
|
Download:
|
| Fig. 1. Electrochemical setup for the CO2 reduction and water purification in one redox system. | |
Bubbling of N2 removed O2 in the electrolyte, which could be recorded in the CV measurements from 0 to -1.6 V (Fig. 2a). Saturation of CO2 by continuous bubbling into the Na2SO4 electrolyte caused an obvious reduction peak at around -1.2 V in the CV measurement as shown in Fig. 2a, which means CO2 could be well reduced on BDD surface. Meanwhile, CV measurements were also employed to monitor KHP in the oxidation side (Fig. 2b). Along with the electrolysis process on the BDD cathode, KHP was almost decomposed on the BDD anode because the KHP oxidation peak drastically decreased. Noting that KHP exhibited a very high oxidation potential (around 2.0 V), which could be well detected on the BDD electrode while could be hardly detected on other carbon or metal electrodes. This advantage is due to a wide potential window of BDD material that avoids the O2 evolution reaction (positive than 2.0 V) for competition of KHP oxidation. A simultaneous reduction of CO2 and oxidation of KHP process completely shows the distinct advantages of BDD electrodes in environmental technology.

|
Download:
|
| Fig. 2. (a) Cyclic voltammograms recorded on the BDD electrode when bubbling with N2 and CO2 in the cathodic side; (b) CVs for monitoring the KHP concentrations in the anodic side. | |
In this research we focus on the influence of the BDD-BDD system on the formaldehyde production from CO2. The reaction products in the cathodic side are mainly formaldehyde, with small amount of formic acid and hydrogen, which have been identified by High performance liquid chromatography (HPLC). A DHP (2, 4-dinitrophenyl)-based HPLC method was used to quantify the generated formaldehyde concentration. For comparison, the electrolysis process for CO2 reduction without KHP was also performed under the same conditions (-1.2 V electrolysis), which was used to evaluate the contribution of the oxidation side.
The HPLC measurement for formaldehyde detection is indicated in Fig. 3a. The concentration of formaldehyde (Fig. 3b) in the presence of KHP was higher than that in the absence of KHP electrolysis, which suggests that KHP degradation process may facilitate the formation of formaldehyde from CO2 on BDD electrodes.

|
Download:
|
| Fig. 3. Comparison of (a) Formaldehyde concentrations in the BDD-BDD system in the presence/absence of KHP by means of HPLC method; (b) Formaldehyde production on the BDD-BDD system in the presence/absence of KHP and a Platinum electrode; and (c) Faradaic efficiency of the formaldehyde formation (black) and KHP consumption (blue) depending on the potential in the BDD-BDD system. | |
On the other hand, electrochemical experiment using a Platinum (Pt) electrode as the anode was carried out for comparison as well as for understanding the effect of using BDD films. This platinum electrode, with the same area of the BDD film, was used for the CO2 reduction in equal condition of the BDD-BDD system in the presence of KHP half-reaction part. The formaldehyde amount formed in the BDD-Pt system was much lower than that on the BDD-BDD system (Fig. 3b). This could be attributed to the narrower potential window potential of platinum material in aqueous solutions even it has highly chemical stability.
In the anodic side, generation of OH· radicals on the BDD surface with a one-electron exchange process between the BDD and the solvent takes places [3]. Only organic species that diffuse to the BDD surface can be destroyed by the hydroxyl radicals. The degradation rate of KHP by these hydroxyl radicals is very fast, and the oxidation reaction takes place close to the anode surface. Owing to its H-terminated surface, BDD has the advantage to avoid absorbing organic molecules on them, which provides a high efficiency in the generation of OH· to destroy organic wastes when applied a positive potential. The presence of Na2SO4 favors the quick formation of OH· on BDD surface when a higher voltage is applied. OH· radicals have a very high potential only lower than fluorine, which can transform many organic into H2O and CO2 [3, 12]. The organic compound (R) degradation process can be briefly described as the follow Reaction (2) [13]:

|
(2) |
Due to the electro-neutrality principle, CO2 reduction in the photocatalytic process has to be balanced at the same oxidation reaction at the anodic side. The introduction of KHP oxidation allows the CO2 reduction process at a rate-controlled step. For the oxidation of KHP at the BDD anode it is important that production of electrons as well as their transport through the membrane to the cathode side is effective [7]. In the view of energy saving, wastewater should be considered as a carrier of energy and resources at the level of the sustainable development [14]. Energy balance with CO2 production from KHP is somewhat compensate that consumed in CO2 reduction. On the other hand, the partial pressure of CO2 in the BDD-BDD system is a little higher than that of BDD-metal systems, which may facilitate propelling the CO2 diffusing onto the BDD surface in the reduction side.
Comparison of Faradaic efficiency for the formaldehyde formation obtained through the electrochemical reduction of CO2 with respect to the potential has been made, as shown in Fig. 3b. From -1.8 V there is a noticeable increase in the Faradaic efficiency for the formaldehyde formation over voltages along the positive direction to -1.2 V (42%), and then the Faradaic efficiency decreases with the voltage increases. The highest peak of the Faradaic efficiency is located at -1.2 V, which is in excellent agreement with the CV results in Fig. 2. The lower Faradaic efficiency at higher negative potential may be caused by the electrolyte decomposition that suppresses the CO2 reduction [10].
Meanwhile, the KHP consumptions at various potentials were measured as indicated in Fig. 3c. More KHP was consumed at more negative potential. This is because when more electrons are applied for the reduction on the cathode, a higher oxidation tendency is induced on the anode. Even at -1.2 V the KHP oxidation could not reach the highest level, oxidant current could still generate the BDD electrode.
Electrochemical reduction of CO2 in aqueous solution at most carbon and metal electrodes, the major products are carbon monoxide, formic acid, methane, ethylene, and methanol [5, 15]. Until now the production of formaldehyde from CO2 by electrochemical reduction with a high yield is still difficult. Previous works of our group found that a carbon electrode with more sp2-carbon showed a low Faradaic efficiency in the electrochemical reduction of CO2. The sp3-carbon of BDD plays an important role in formaldehyde production. On BDD surface CO2 follows a reduction process from formic acid to formaldehyde. The whole process undergoes a fourelectron reduction pathway. Effective suppression of hydrogen is of significance for energy saving [16, 17]. In this research, the wide potential window of BDD reduces the evolution for both hydrogen and oxygen, thus promoting the CO2 conversion and KHP degradation efficiency simultaneously. In a short summary, the whole reaction in this system may be expressed as "organic waste conversion into useful hydrocarbons". The stability of this treatment system was evaluated and it was found the at least 20 h duration of working time for each piece of BDD film for the operation. After that, the BDD electrodes could regenerate activity if were treated by hydrogen plasma or electrochemical circulated in acid electrolyte.
In conclusion, for the first time a BDD-BDD system was developed in the simultaneous conversion of CO2 and wastewater purification in one electrochemical compartment. In 0.1 mol/L Na2SO4 electrolyte, it was found higher amount of formaldehyde was produced from CO2 on the BDD cathode in this novel BDD-BDD system than that in conventional BDD-Pt electrochemistry. When using KHP as the waste material, besides KHP was almost decomposed on the BDD anode, the formaldehyde production in the cathodic side was promoted. The Faradaic efficiency was estimated at various potentials for the formaldehyde formation from CO2 and an optimal working potential was found located at around -1.2 V. The results indicated that this novel method opens the possibility of integrating the CO2 electrochemical reduction process and wastewater purification into one process. This system is of significant importance for waste treatment as well as energy and material recycling, thus is expected to be developed into a smart device for environmental protection and CO2 reusing.
AcknowledgmentsThis work was supported by the scholarship under the Sichuan University Scholarship Fund allocated by the Ministry of Education to pursue his research as a visiting scholar overseas, and the Experimental Technology Project (No. 20170209) of Sichuan University.
| [1] |
E.V. Kondratenko, G. Mul, J. Baltrusaitis, G.O. Larrazábal, J. Pérez-Ramírez, Energy Environ. Sci. 6 (2013) 3112-3135. DOI:10.1039/c3ee41272e |
| [2] |
B.Y. Chen, S.Q. Liu, J.Y. Hung, T.J. Shiau, Y.M. Wang, Aerosol Air Qual. Res. 13 (2013) 266-274. DOI:10.4209/aaqr.2012.05.0122 |
| [3] |
A. Fujishima, Y. Einaga, T.N. Rao, D.A. Tryk, Diamond Electrochemistry. Tokyo: Elsevier, 2005: pp. 449-499.
|
| [4] |
G. Centi, S. Perathoner, Catal. Today 148 (2009) 191-205. DOI:10.1016/j.cattod.2009.07.075 |
| [5] |
D.T. Whipple, P.J.A. Kenis, J. Phys. Chem. Lett. 1 (2010) 3451-3458. DOI:10.1021/jz1012627 |
| [6] |
Y. Hori, Mod. Asp. Electrochem. 42 (2008) 89-189. DOI:10.1007/978-0-387-49489-0 |
| [7] |
S.M.A. Kriescher, K. Kugler, S.S. Hosseiny, Y. Gendel, M. Wessling, Electrochem. Commun. 50 (2015) 64-68. DOI:10.1016/j.elecom.2014.11.014 |
| [8] |
D. Kim, J. Resasco, Y. Yu, A.M. Asiri, P.D. Yang, Nat. Commun. 5 (2014) 4948. DOI:10.1038/ncomms5948 |
| [9] |
D.B. Luo, J.F. Zhi, Electrochem. Commun. 11 (2009) 1093-1096. DOI:10.1016/j.elecom.2009.03.011 |
| [10] |
K. Nakata, T. Ozaki, C. Terashima, A. Fujishima, Y. Einaga, Angew. Chem. Int. Ed. 53 (2014) 871-874. DOI:10.1002/anie.201308657 |
| [11] |
M. Panizza, E. Brillas, Ch. Comninellis, J. Environ. Eng. Manag. 18 (2008) 139-153. |
| [12] |
D.B. Luo, D.C. Ma, L.Z. Wu, J.F. Zhi, Int. J. Electrochem. Sci. 13 (2018) 5904-5922. |
| [13] |
M.A. Rodrigo, P.A. Michaud, I. Duo, et al., J. Electrochem. Soc. 148 (2001) D60-D64. DOI:10.1149/1.1362545 |
| [14] |
X.D. Hao, F.G. Qiu, M.C.M. van Loosdrecht, Int. J. Environ. Pollut. 45 (2011) 237-248. DOI:10.1504/IJEP.2011.039099 |
| [15] |
S. Kaneco, K. Iiba, S. Suzuki, K. Ohta, T. Mizuno, J. Phys. Chem. B 35 (1999) 7456-7460. |
| [16] |
P.K. Jiwanti, K. Natsui, K. Nakata, Y. Einaga, RSC Adv. 6 (2016) 104. |
| [17] |
N. Roy, Y. Shibano, C. Terashima, et al., ChemElectroChem 3 (2016) 1044-1047. DOI:10.1002/celc.201600105 |
 2019, Vol. 30
2019, Vol. 30 

