Polyacrylonitrile (PAN) has been widely used not only in textile industry but also as an important precursor to produce carbon fibers [1-3]. PAN-based carbon fibers are superior composite materials in view of their high strength and stiffness, light weight as well as low cost [4]. It is known that the properties of PAN precursor fibers and resulting carbon fibers are improved with the increase in the molecular weight of PAN [5-7]. PAN with molecular weight higher than5 × 105 g/mol is essential to produce carbon fiberswith strength ofnolessthan4.5 GPa and elastic modulus of no less than 450 GPa [8].
The commercially available PAN is primarily prepared via radical polymerization [9]. Radical polymerization of acrylonitrile (AN) to get high molecular weight (>106 g/mol) PAN has been studied extensively [10-15]. However, the high molecular weight PAN obtained via radical polymerization is usually isolated from the aqueous solution and shows defects in forms of branching and enaminonitrile formation as reported [16]. Moreover, the spinning solution of carbon fiber can only be prepared by dissolving the dried PAN which increasing the process complexity and energy expenditure [17].
PAN could also be synthesized via anionic polymerization procedure. Anionic polymerization takes some advantages such as high rate, high yield, clean polymerization system and getting monodisperse polymers over radical polymerization. Many kinds of initiators such as metal alkyls [18, 19] and alkali metal alkoxides [20-22] have been explored for the anionic polymerization of AN. Anionic homogeneous polymerization to prepare high molecular weight PAN (>5 × 105 g/mol) has been achieved using lithium alkoxides as initiators in N, N-dimethylformamide (DMF) and the side reactions which are characteristic features of AN anionic polymerization initiated by metal alkyls can be obviated [19, 23, 24]. Korotkov et al. [25] reported the anionic polymerization of AN initiated by lithium butyl(dimethylamino)carbinolate and high molecular weight PAN (>6 × 105 g/mol) could be obtained. Novosselova et al. [26] utilized lithium trimethylcarbinolate to prepare PAN with molecular weight higher than 106 g/mol. Recently, they reported the anionic polymerization of AN initiated by lithium 1, 2-bis(dimethylamino)-2-oxoethanolate and PAN with molecular weight ranging from 6.5 ×105 g/mol to 8.4 ×105 g/mol could be prepared in DMF [8].
Lithium amides are commercially available bases and have been widely used in organic synthesis [27]. They have been used as effective initiators for the anionic polymerizations of methacrylates [28-32]. However, there is no report about their utility as the anionic initiators of AN to our knowledge. Theoretically, lithium amides can be superior anionic initiators of AN because they are less nucleophilic strong bases. Moreover, the sterically hindrance of lithium amides is beneficial to suppress side reactions such as the nucleophilic attack of initiator or propagating end toward the cyano group of AN. In this work, the anionic polymerization of AN initiated by various lithium amides was investigated. The aim is to prepare PAN with high molecular weight and discover whether the use of lithium amides could obviate side reactions.
DMF and AN were kept over 4 Å molecular sieves for more than one week. DMF was dried with calcium dihydride (CaH2) overnight and distilled in vacuum just before use. AN was further purified by fractional distillation over CaH2 under argon and redistilled just before use. Hexane and tetrahydrofuran (THF) were purified by distillation under argon in the presence of a purple sodium/ benzophenone complex. Butyl lithium was prepared from n-butyl chloride and lithium in hexane according to the conventional method and the concentration was determined by the double titration method [33]. Diisopropylamine, diethylamine, hexamethyldisilazane, dicyclohexylamine and 2, 2, 6, 6-tetramethylpiperidine were dried over CaH2 and distilled before use.
As a typical procedure for the polymerization of AN initiated by lithium amides, the procedure for entry 5 in Table 1 was conducted as follows. A THF solution (20 mL) of diisopropylamine (0.18 mL, 1.25 mmol) was placed in a Schlenk bottle under argon and cooled down to -30 ℃. Butyl lithium (0.92 mL of a 1.36 mol/L solution in hexane, 1.25 mmol) was added under stirring. The resulting solution of lithium diisopropylamide (LDA) was used as initiator solution. Purified DMF (20 mL) and AN (2.5 mL, 38 mmol) were transferred to another Schlenk bottle under argon. The mixture was stirred and cooled down to -60 ℃, and then the LDA (0.1 mL of a 6.25 ×10-2 mol/L solution in THF, 6.25 mmol) was injected into the reaction mixture rapidly with syringe under vigorous stirring. The polymerization was quenched by 1 mL of acetic acid in DMF (1:10, v/v) after 5 min. The reaction mixture was poured into water (200 mL) and the polymer was isolated by filtration, washed with water and dried.
|
|
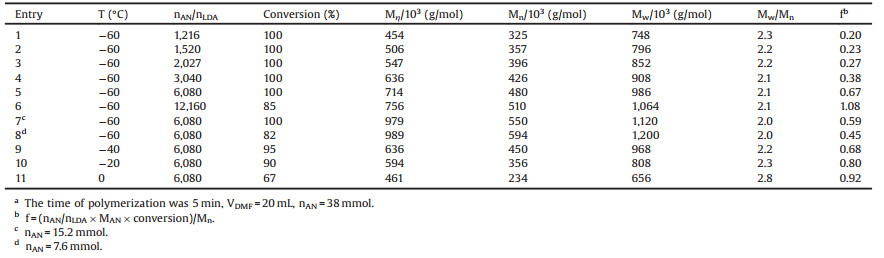
Table 1 Anionic polymerization of AN initiated by LDA.a |
The intrinsic viscosity (η) of the obtained PAN was determined in an Ubbelohde capillary viscometer at 25 ℃ in DMF. The viscosity-average molecular weight (Mη) of obtained PAN was calculated from the Mark-Houwink equation [34]:

|
The number-average molecular weight (Mn), weight-average molecular weight (Mw) and molecular weight distribution (MWD, Mw/Mn) of PAN were measured by gel permeation chromatography (GPC) (Elite P230) equipped with a PolarGel-M column (7.5 mm × 300 mm, Agilent) and a refractive index detector (Shodex RI201H). DMF with 10 mmol/L of LiBr was used as eluent at a flow rate of 1 mL/min at 40 ℃. The GPC samples were prepared at concentration of 1 mg/mL with an injection volume of 50 μL and calibrated with linear polystyrene (PS) standards. The molecular weight of PAN was calculated as MGPC, PS/2.5 according to literature [35] in the following discussion.
Lithium diisopropylamide (LDA) is one of the most typical lithium amides. It is a sterically hindered, less nucleophilic strong base and relative cheap as well. The effects of different reaction parameters on the anionic polymerization of AN were investigated using LDA as initiator.
In order to obtain high molecular weight PAN, the anionic polymerization of AN initiated by LDA with different nAN/nLDA were carried out at -60 ℃ and the reaction results are presented in Table 1 (entries 1–6). It can be seen that the molecular weight of obtained PAN increases as the increase of nAN/nLDA and this is in accordance with the general rule of polymerization. PAN with Mw higher than 106 g/mol could be prepared with LDA as initiator.
The initiator efficiency (f) of LDA was calculated and listed as well. It can be seen that f increases as the increase of nAN/nLDA. This can be explained according to the high propensity of LDA to aggregate [36]. An assumption can be suggested that LDA exists mainly as aggregated form in the polymerization solution in this study and equilibrium may exist between the aggregated and monomeric form of LDA. When nAN/nLDA is relative low, a relative larger amount of initiator solution is added, the monomer AN has been consumed up before all the aggregated LDA is transformed to monomeric form. Only a part of LDA actually participates in the polymerization. The initiator efficiency is lower as a result. When nAN/nLDA is large, such as entry 6 in Table 1, the initiator efficiency can reach 100%, which indicates that all the LDA has transformed to monomeric form and used up.
The MWD of PAN obtained above is mainly in the range of2.1–2.3. This may be due to two reasons. Firstly, the polymerization rate is very fast and the reaction mixture becomes viscous rapidly after the addition of initiator. The mass and heat transfers will become worse with the increase of polymerization solution viscosity, which causes the broadening of MWD [37]. Secondly, the rate of the deaggregation of LDA to monomeric form and the rate of initiation are slower than the rate of chainpropagation, thus leading to broad MWD. In order to get high molecular weight PAN with narrower MWD, the polymerization was explored with lower monomer concentration and the results are presented in Table 1 (entries 5, 7, 8). It can be seen that diluted solution of AN favors the formation of higher molecular weight PAN with slightly decreased MWD. So, it seems that the second reasonis themajor reason of wide MWDand the bad heat and mass transfers only have minor effects.
From the point of operation simplification and energy conversation, excessively low temperature is barrier to practical application and industrial production for anionic polymerization. In order to investigate the effect of temperature on anionic polymerization in this study, the polymerizations of AN in DMF initiated by LDA within the temperature range from -60 ℃ to 0 ℃ were examined and the reaction results are summarized in Table 1 (entries 5, 9–11).
It can be observed that, the drop in conversion is accompanied by a decrease in molecular weight and a broadening in MWD as elevated temperature. When the polymerization temperature is below -20 ℃, the conversion of AN is still higher than 90%, the molecular weight of obtained PAN is higher than 8 × 105 g/mol and the MWD is lower than 2.3. But when the polymerization proceeds at 0 ℃, the conversion of AN drops to 67% and the MWD of obtained PAN is as high as 2.8. This may be due to the growing contribution of side reactions with elevated temperature.
It also shows that f increases as the increase of temperature. This can be explained by the increasing percentage of monomeric form of LDA with elevated temperature, thus resulting higher f.
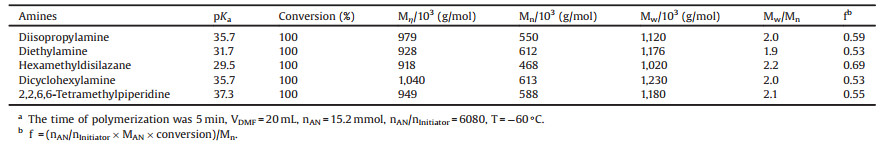
Besides LDA, lithium amides with steric hindered groups and strong basicity derived from diethylamine, hexamethyldisilazane, dicyclohexylamine, and 2, 2, 6, 6-tetramethylpiperidine have been used to initiate the polymerization of AN in DMF in this study. The reaction results are listed in Table 2. Table 2 shows that all the lithium amides selected in this study are efficient anionic initiators to get PAN with high molecular weight. PAN with Mw ranging from 1.02 ×106 g/mol to 1.23 ×106 g/mol (Mw/Mn = 1.9–2.2) can be prepared at -60 ℃.
|
|
Table 2 Anionic polymerization of AN initiated by lithium amides derived from different amines.a |
The aggregation of lithium amides has been described in a numbers of publications [38-40]. The values of initiator efficiency (f = 0.53–0.69) listed in Table 2 also indicate the presence of aggregated lithium amides.
The situations initiated by other lithium amides are similar to that of LDA. It can be concluded that the large steric hindrance and low nucleophilicity and of lithium amides are efficient pathways to protect the anionic polymerization from oligomerization termination at early stages of polymerization compared with metal alkyls. From the pKa values of the conjugated amines [41, 42] listed in Table 2, it can be seen that those lithium amides are of strong basicity. Accordingly, the activity of the active anions and the polymerization rate may be very high, which benefits the preparation of high molecular weight PAN.
The polymerization of AN initiated by lithium amides can proceed in a homogeneous manner in DMF. Once the initiator solution was injected into the polymerization system, the reaction mixture became viscous rapidly. The monomer conversion could reach 100% within 1 min (Fig. S1 in Supporting information).
Acrylonitirle is an active vinyl monomer for anionic polymerization and the mechanism of acrylonitrile initiated by lithium amides is typical anionic polymerization mechanism. The obvious cyano absorption peak at 2240 cm-1 in IR spectrum (Fig. S2 in Supporting information) indicated that the polymerization occurred via lithium amide nucleophilic attack at the carbon-carbon double bond instead of carbon-nitrogen triple bond. The mechanism was confirmed by the presence of amino end group in the 1H NMR spectrum (Fig. S3 in Supporting information).
No low molecular weight fraction extracted from acetone was found in the obtained PAN, which indicated that no oligomerization occurred during the polymerization procedure [19].
Neither gelation nor color-change phenomenon was observed for the polymerization solution, which indicated that crosslinking and cyclization are not obvious [43]. The insignificant contribution of side reactions for the polymerization initiated by lithium amides could also be confirmed by the 1H NMR spectra (Fig. S4 in Supporting information).
Lithium amides have been proved to be effective anionic initiators for the synthesis of high molecular weight PAN. High mole ratio of monomer to initiator, suitable monomor concentration and low temperature are favorable for the synthesis of high molecular weight PAN. PAN with high molecular weight (1.02 ×106 g/mol < Mw < 1.23 ×106 g/mol, Mw/Mn = 1.9–2.2) could be prepared utilizing lithium amides derived from diisopropylamine, diethylamine, hexamethyldisilazane, dicyclohexylamine, and 2, 2, 6, 6-tetramethylpiperidine as initiators. The anionic polymerization of AN initiated by lithium amides proceeded in a homogeneous manner in DMF and insignificant contribution of side reactions was confirmed. Works on obtaining PAN with high molecular weight as well as narrow MWD will be continued.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.040.
| [1] |
A.K. Gupta, D.K. Paliwal, P. Bajaj, J. Macromol. Sci. Rev. Macromol. Chem. Phys. C 31 (1991) 1-89. |
| [2] |
P. Rajalingam, G. Radhakrishnan, J. Macromol, J. Macromol. Sci. Rev. Macromol. Chem. Phys. C 31 (1991) 301-310. DOI:10.1080/15321799108021925 |
| [3] |
C.D.E. Lakeman, G. Pan, N. Muto, et al., Mater. Lett. 13 (1992) 330-335. DOI:10.1016/0167-577X(92)90063-P |
| [4] |
J.L. Figueiredo, C.A. Bernardo, R.T.K. Baker, K.J. Huttinger, Carbon fibers, filaments, and composites, in: D.J. Johnson (Ed.), Structure and properties of carbon fibres, Kluwer Academic Publishers Dordrecht, Boston, London, 1990, pp. 119-146.
|
| [5] |
J.S. Tsai, C.H. Lin, J. Appl. Polym. Sci. 42 (1991) 3045-3050. DOI:10.1002/app.1991.070421124 |
| [6] |
A.V. Volokhina, Fibre Chem. 34 (2002) 1-9. DOI:10.1023/A:1015564704979 |
| [7] |
X. Lin, C.G. Wang, M.J. Yu, Z.T. Lin, Adv. Mater. Res. 781- 784 (2013) 2609-2613. |
| [8] |
A.V. Novoselova, V.V. Shamanin, L.V. Vinogradova, Polym. Sci. Ser. B 51 (2009) 205-211. DOI:10.1134/S156009040907001X |
| [9] |
H. Kricheldorf, N. Oskar, G. Swift, Handbook of Polymer Synthesis, in: O. Nuyken, G. Lattermann (Eds.), Acrylonitrile, Marcel Dekker Inc., New York, 1992, pp. 280-296.
|
| [10] |
T. Kobashi, S. Takagi, Patent, JP 59-191704, 1984.
|
| [11] |
F. Ueda, H.Tanaka, Patent, JP 63-182317, 1988.
|
| [12] |
Y. Nishihara, Y. Hosako, T. Tabuchi, H. Matsusue, Patent, JP 03-210309, 1991.
|
| [13] |
K. Nishida, K. Shigeoka, H. Matsusue, Y. Nishihara, Patent, JP 05-025223, 1993.
|
| [14] |
C. Zhang, R.D. Gilbert, R.E. Fornes, J. Appl. Polym. Sci. 58 (1995) 2067-2075. DOI:10.1002/app.1995.070581119 |
| [15] |
E.A. Morris, M.C. Weisenberger, S.B. Bradley, et al., Polymer 55 (2014) 6471-6482. DOI:10.1016/j.polymer.2014.10.029 |
| [16] |
M. Minagawa, J. Polym. Sci. Part A:Polym. Chem. 18 (1980) 2307-2322. DOI:10.1002/pol.1980.170180724 |
| [17] |
B.A. Newcomb, Compos. Part A:Appl. Sci. Manuf. 91 (2016) 262-282. DOI:10.1016/j.compositesa.2016.10.018 |
| [18] |
B.A. Feit, D. Mirelman, A. Zilkha, J. Appl. Polym. Sci. 9 (1965) 2459-2474. DOI:10.1002/app.1965.070090710 |
| [19] |
B.L. Erussalimsky, I.G. Krasnoselskaya, V.N. Krasulina, A.V. Novoselova, E.V. Zashtsherinsky, Eur. Polym. J.. 6 (1970) 1391-1396. DOI:10.1016/0014-3057(70)90071-6 |
| [20] |
A. Zilkha, B.A. Feit, J. Appl. Polym. Sci. 5 (1961) 251-260. DOI:10.1002/app.1961.070051502 |
| [21] |
B.A. Feit, A. Zilkha, J. Appl. Polym. Sci. 7 (1963) 287-300. DOI:10.1002/app.1963.070070126 |
| [22] |
B.A. Feit, J. Wallach, A. Zilkha, J. Polym. Sci. Part A:Gen. Pap. 2 (1964) 4743-4755. DOI:10.1002/pol.1964.100021105 |
| [23] |
A.V. Novoselova, B.L. Yerusalimskii, V.N. Krasulina, Y.V. Zashcherinskii, Polym. Sci. USSR 13 (1971) 99-104. DOI:10.1016/0032-3950(71)90223-1 |
| [24] |
B.L. Erussalimsky, Makromol. Chem. 182 (1981) 911-915. DOI:10.1002/macp.1981.021820321 |
| [25] |
V.N. Korotkov, A.V. Krasulina, Vysokomol. Soedin. A 13 (1971) 2661-2666. |
| [26] |
A.V. Novosselova, G. Orlova, B.L. Erussalimsky, H.J. Adler, W. Berger, Acta Polym. 36 (1985) 599-602. DOI:10.1002/actp.1985.010361103 |
| [27] |
B. Luisi, V. Capriati, Lithium compounds in organic synthesis, in: S.O.N. Lill (Ed.), Lithium alkoxides and lithium amides, Wiley-VCH, Weinheim, Germany, 2014, pp. 41-43.
|
| [28] |
T.E. Long, R.A. Guistina, B.A. Schell, J.E. McGrath, J. Polym. Sci. Part A:Polym. Chem. 32 (1994) 2425-2430. DOI:10.1002/pola.1994.080321303 |
| [29] |
S. Antoun, P. Teyssie, R. Jerome, J. Polym. Sci. Part A:Polym. Chem. 35 (1997) 3637-3644. |
| [30] |
S. Antoun, P. Teyssie, R. Jerome, Macromolecules 30 (1997) 1556-1561. DOI:10.1021/ma961394a |
| [31] |
S.A. Couper, R.E. Mulvey, D.C. Sherrington, Eur. Polym. J. 34 (1998) 1877-1887. DOI:10.1016/S0014-3057(98)00026-3 |
| [32] |
E. Ihara, N. Omura, S. Tanaka, T. Itoh, K. Inoue, J. Polym. Sci. Part A:Polym. Chem. 43 (2005) 4405-4411. |
| [33] |
H. Gilman, F.K. Cartledge, J. Organomet. Chem. 2 (1964) 447-454. DOI:10.1016/S0022-328X(00)83259-3 |
| [34] |
R.L. Cleland, W.H. Stockmayer, J. Polym. Sci. 17 (1955) 473-477. DOI:10.1002/pol.1955.120178602 |
| [35] |
H. Dong, W. Tang, K. Matyjaszewski, Macromolecules 40 (2007) 2974-2997. DOI:10.1021/ma070424e |
| [36] |
A.S. Galiano-Roth, D.B. Collum, J. Am. Chem. Soc. 111 (1989) 6772-6778. DOI:10.1021/ja00199a042 |
| [37] |
F. Bally, C.A. Serra, V. Hessel, G. Hadziionanou, Macromol. React. Eng. 4 (2010) 543-561. DOI:10.1002/mren.v4:9/10 |
| [38] |
F.E. Romesberg, J.H. Gilchrist, A.T. Harrison, D.J. Fuller, D.B. Collum, J. Am. Chem. Soc. 113 (1991) 5751-5757. DOI:10.1021/ja00015a032 |
| [39] |
F.E. Romesberg, M.P. Bernstein, J.H. Gilchrist, et al., J. Am. Chem. Soc. 115 (1993) 3475-3483. DOI:10.1021/ja00062a010 |
| [40] |
E. Weiss, Angew. Chem. Int. Ed. 32 (1993) 1501-1523. |
| [41] |
R.R. Fraser, T.S. Mansour, J. Org. Chem. 49 (1984) 3442-3443. DOI:10.1021/jo00192a059 |
| [42] |
H. Ahlbrecht, G. Scheneider, Tetrahedron 42 (1986) 4729-4741. DOI:10.1016/S0040-4020(01)82054-8 |
| [43] |
A. Ottolenghi, A. Zilkha, J. Polym. Sci. Part A:Gen. Pap. 1 (1963) 687-703. DOI:10.1002/pol.1963.100010209 |
 2019, Vol. 30
2019, Vol. 30