Although C18-functionalized reverse phase (RP) stationary phase (SP) has dominated the applications of high performance liquid chromatography, it still has some drawbacks. Such phases generally possess single interaction mechanism and thus can provide effective retention only towards the hydrophobic analytes. The hydrophilic or polar analytes are always less retained on RP column [1, 2]. In addition, such phase cannot bear high content aqueous solution due to the collapse of aliphatic chain [3]. These drawbacks can be addressed well by the use of C18-aqueous SP [4, 5] or mixed-mode SPs [6-8], in which multiple interactions (at least two interactions) between the SPs and analytes exist, contributing to the retention of the analytes to provide easy manipulation of the separation selectivity compared to a single one [9-11]. A typical application is for the simultaneous analysis of active pharmaceutical ingredients (APIs) and counterions, which is an important assay in pharmaceutical analysis [12]. Due to wide variety of ionizable substituents and hydrophobicity of these pharmaceutical-related molecules, it is challenging to simultaneously separate APIs and respective counterions on a RP column. The latter are always much less retained on RP column.
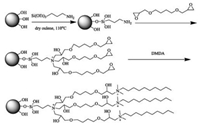
Here we described the synthesis of a novel tri-mode stationary phase (TMSP) consisting of (RP)/hydrophilic interaction (HILIC)/ion exchange (IEX) starting from propylamine on silica by amine-epoxy reactions with 1, 4-butanedioldiglycidyl ether (BDDE) and tertiary amines (N, N-dimethyldecylamine, DMDA). Its retention mechanism was explored and a preliminary application was also demonstrated.
The synthesis route of TMSP was shown in Fig. 1. Prior to use, silica gel was activated by refluxing with the 3 mol/L HCl for 12 h. After filtration, the silica gel was subsequently washed with water and methanol, and then dried at 120 ℃. The treated silica gel (3 g) above was aminated by reaction with 3-aminopropyltriethoxysilane (3 mL) and the pyridine as a catalyst in anhydrous toluene (50 mL) for 12 h at 110 ℃ under nitrogen atmosphere. After filtration, the silica gel was subsequently washed with toluene, methanol and dried at 70 ℃. The obtained gels were stirred with BDDE in water (100 mL, 0.3 mol/L) at 70 ℃ for 1 h. After filtration, the residue was rinsed with pure water. Then, 8 mL DMDA were added to the modified silica gel and reacted in methanol for 1 h at 50 ℃. Finally, the gels was rinsed with water and methanol, and then dried at 70 ℃ to obtain the final TMSP.
|
Download:
|
| Fig. 1. Synthesis route of TMSP. | |
The obtained TMSP were packed into a stainless-steel column (4.6 mm × 150 mm) with the packing pressure of 50 MPa by using methanol as the packing and slurry solvent.
The TMSP was characterized by elemental analysis, IR spectrometry and Zeta-potential analysis, as provided in Supporting information (Table S1, Figs. S1 and S2 in Supporting information, respectively). In Table S1, it can be seen that an obvious variation of carbon and nitrogen contents of TMSP relative other gels were observed. In addition, the peaks at 2939, 2876 and 1456 cm-1 in the IR spectrum were attributed to stretching vibration and bending vibration of alkyl. The positively charge of TMSP in the tested pH range was confirmed by the Zeta-potential measurement (Fig. S2). These characterizations indicated that the functional groups were successfully grafted onto the surface of silica gel.
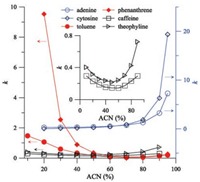
The retention mechanism of TMSP was investigated by varying the acetonitrile content and salt concentration. Firstly three kinds of analytes including polar (adenine, cytosine), non-polar (toluene, phenanthrene), and basic (caffeine, theophyline) analytes were chosen to explore their retention dependence on the acetonitrile content in the mobile phase. As shown in Fig. 2, the retention of both polar analytes increases with the increase of acetonitrile (ACN) content in the tested range (20%–95%), exhibiting typical hydrophilic interaction behavior. By contrast, the retention behavior of both non-polar analytes shows typical RP mechanism in the range of 10%–80% acetonitrile. When further increasing acetonitrile content up to 95%, their retention slightly increases, showing very weak HILIC behavior. Presently such weird phenomenon is unclear if considering their highly hydrophobic property. But it suggests the special selectivity of such column. In addition, the retention of both basic analytes exhibits obviously "U-shape" curve with the minimum k occurring at the point of the acetonitrile content of 50%, suggesting RP and HILIC dual retention mechanism.
|
Download:
|
| Fig. 2. Effect of ACN content on the retention of analytes on TMSP. Mobile phase: 5 mmol/L NH4FA, pH 6.53 with varying acetonitrile content; UV detection: 254 nm; injection volume: 2 μL; column temperature: 30 ℃. | |
The addition of salt in the mobile phase is always required in the HILIC mode aiming to improve the peak shape or retention of the analytes [13, 14]. Herein, the influence of salt concentration on retention was studied by varying NH4FA concentrations in the mobile phase (pH kept at 3.68) by choosing four polar analytes (as shown in Fig. S3 in Supporting information). The retention of 3, 5- dinitrobenzoic acid decreased with the increase of salt concentration, indicating that ion exchange interaction dominated in the retention of negative organic acid and the quaternary ammonium groups on the TMSP. The other neutrally charged analytes (adenine, caffeine, and phenanthrene) barely have electrostatic interaction with TMSP. Therefore their retention was almost constant with varying salt concentration.
HILIC SP often suffer from column bleeding (or de-wetting) in highly aqueous conditions [3, 15], probably due to the hydrolysis of siloxane linkage [6]. As mentioned above, C18 RP SP also suffers from the collapse under highly aqueous solution [3]. Here, to validate the compatibility of the TMSP with 100% aqueous mobile phase, the stop-flow test was performed according to the previous description [4]. Briefly, uracil and adenine were chosen to be the model for probing such test and 50 mmol/L NH4FA buffer was used as the mobile phase. TMSP column was washed with 100 column volumes of acetonitrile, followed by the equilibration with 100 column volumes of aqueous mobile phase, then was equilibrated with the mobile phase above for 20 min, then performing the injection and data acquisition. After each data acquisition, the flow was stopped for 30 min before starting the next cycle. As shown in Fig. S4 (Supporting information), the column provides stable retentions of analytes in 100% aqueous mobile phase, exhibiting an excellent compatibility with highly aqueous mobile phase. This should result from the existence of quaternary ammonium and hydroxyl groups in the TMSP structure for the contribution to resistant to de-wetting.
A drawback of silica-based polar phases is prone to bleeding owing to silica dissolution in aqueous solution [16]. If mass- dependent is employed, e.g., such as mass spectrometer (MS) and evaporative light scattering detector (ELSD), such bleeding is always reflected directly by high background signal or large noise [17]. Thus the bleeding level can be evaluated by measuring the background signal of mass-dependent detector. Here the bleeding of TMSP column was measured. To facilitate comparison, several commercial columns were selected. As shown in Fig. S5 (Supporting information), although the bleeding of TMSP is higher than that of pure RP column (C18), it really shows much lower level than other silica-based polar SPs (e.g., ~12-fold lower than Spherisorb NH2 column), which should result from the protection of long alkyl chain.
The running stability of TMSP was also evaluated in a mobile phase consisting of 40% acetonitrile-20% 400 mmol/L ammonium formate (pH 5.94)-40% water. The retention of four model analytes was investigated by continuous operation with the mobile phase over 2880 column volume. The relative standard deviation (RSD) of the retention times (tR) and the plate counts were in the range of 0.34%–0.45% and 0.32% for 3, 5-dinitrobenzoic acid, respectively (shown in Fig. 3). Intra-day and inter-day RSDs of the retention times were in the range of 0.03%–0.11% (n = 6) and 0.28%–0.48% (n = 3), respectively, indicating excellent running stability of TMSP.

|
Download:
|
| Fig. 3. Stability of TMSP column. Mobile phase: H2O/ACN/NH4FA (400 mmol/L, pH 5.94) (40/40/20, v/v/v); analytes: A, caffeine; B, adenine; C, phenanthrene; D, 3, 5- dinitrobenzoic acid. Other conditions are same as those of Fig. 2. | |
In view of hydroxyl groups, alkyl chain and quaternary ammonium groups associated with TMSP, it can in principle provide hydrophilic, hydrophobic and anion exchange interaction towards suited analytes. By choosing nucleosides, bases, hydrophobic analytes and inorganic anions as models, the chromatographic performance of TMSP was evaluated, as shown in Figs. S6–8 (Supporting information). In Fig. S6, six nucleosides can be well separated with good efficiency (e.g., the plate count for adenosine is 45740/m) under isocratic mode. Similarly, good separation of three polycyclic aromatic hydrocarbons was also obtained by TMSP (Fig. S7 in Supporting information). If choosing longer alkyl chain, the efficiency may be improved. Fig. S8 (Supporting information) illustrated the separation of five inorganic anions with UV absorption property via a 100% aqueous solution (10 mmol/L Na2SO4). The plate count for bromate was 66253/m, much higher than that of common polymer-based anion exchanger. A typical application for the simultaneous analysis of basic, non-polar and acidic analytes was illustrated on TMSP under gradient elution, as shown in Fig. 4a. The simultaneous separation of APIs and respective counterion was demonstrated in Fig. 4b.

|
Download:
|
| Fig. 4. Separation of complicated analytes. (a) Mobile phase: H2O-ACN-NH4FA (200 mmol/L, pH 4.07) gradient elution: 0–5 min: 10% ACN, 20% NH4FA; 5– 7 min:10%-40% ACN, 20% NH4FA; 7–12 min: 40% ACN, 20% NH4FA; 12-12.1 min: 40% ACN, 20%-45% NH4FA; 12.1–25 min: 40% ACN, 45% NH4FA; UV detection: 254 nm; injection volume: 4 μL; analytes: A: diprophylline, B: theophylline, C: 3-nitroaniline, D: phenanthrene, E: benzophenanthrene, F: cinnamic acid, G: 3, 5-dinitrobenzoic acid. (b) Mobile phase: ACN-NH4FA (200 mmol/L, pH 4.07) (60/40, v/v); ELS detection: nitrogen nebulizer gas 30 psi, tube temperature 50 ℃, gain 10; injection volume, 4 μL. | |
In summary, a dendritic quaternary ammonium-modified silica mixed-mode SP is described. It demonstrated RPLC/HILIC/IEX trimode retention mechanism. Such method can be extended to prepare many kinds of SP due to high reaction activity of the epoxy group terminal, e.g., by introducing longer alkyl chain, it brings stronger RP interaction. Such mixed-mode SP will be useful for a wider range of applications.
AcknowledgmentsThe work was financially supported by the National Natural Science Foundation of China (Nos. 21477037, 21322502) and the Outstanding Young Talent Cultivation Fund of East China University of Science and Technology.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.04.013.
| [1] |
S.C. Churms, J. Chromatogr. A 720 (1996) 75-91. DOI:10.1016/0021-9673(95)00306-1 |
| [2] |
X. Dai, Y. He, Y. Wei, B. Gong, Chin. Chem. Lett. 22 (2011) 245-248. DOI:10.1016/j.cclet.2010.07.036 |
| [3] |
M. Przybyciel, R.E. Majors, LC-GC North Am. 20 (2002) 520-523. |
| [4] |
X. Liu, A. Bordunov, M. Tracy, et al., J. Chromatogr. A 1119 (2006) 120-127. DOI:10.1016/j.chroma.2005.12.097 |
| [5] |
H. Liu, Z. Li, D. Liu, Y. Xue, Z. Shi, J. Chromatogr. A 1443 (2016) 175-180. DOI:10.1016/j.chroma.2016.03.043 |
| [6] |
X. Liu, C. Pohl, J. Chromatogr. A 1191 (2008) 83-89. DOI:10.1016/j.chroma.2007.12.012 |
| [7] |
Q. Wang, Y. Long, L. Yao, et al., Talanta 146 (2016) 442-451. DOI:10.1016/j.talanta.2015.09.009 |
| [8] |
T. Liang, A. Shen, H. Wang, et al., J. Chromatogr. A 1388 (2015) 110-118. DOI:10.1016/j.chroma.2015.02.019 |
| [9] |
M. Lämmerhofer, R. Nogueira, W. Lindner, Anal. Bioanal. Chem. 400 (2011) 2517-2530. DOI:10.1007/s00216-011-4755-3 |
| [10] |
H. Qiu, A.K. Mallik, M. Takafuji, S. Jiang, H. Ihara, Analyst 137 (2012) 2553-2555. DOI:10.1039/c2an35348b |
| [11] |
Y. Li, J. Yang, J. Jin, et al., J. Chromatogr. A 1337 (2014) 133-139. DOI:10.1016/j.chroma.2014.02.044 |
| [12] |
X. Liu, C. Pohl, J. Chromatogr. A 1232 (2012) 190-195. DOI:10.1016/j.chroma.2011.12.009 |
| [13] |
Y. Hou, F. Zhang, X. Liang, et al., Anal. Chem. 88 (2016) 4676-4681. DOI:10.1021/acs.analchem.5b04384 |
| [14] |
Y. Peng, F. Zhang, X. Pan, Y. Hou, B. Yang, RSC Adv. 7 (2017) 21336-21341. DOI:10.1039/C7RA01958K |
| [15] |
T. Enami, N. Nagae, S. Doshi, LC-GC Europe 16 (2003) 418-425. |
| [16] |
Z. Huang, M.A. Richards, Y. Zha, et al., J. Pharm. Biomed. Anal. 50 (2009) 809-814. DOI:10.1016/j.jpba.2009.06.039 |
| [17] |
K. Qian, Y. Peng, F. Zhang, B. Yang, X. Liang, Talanta 18 (2018) 500-504. |
 2019, Vol. 30
2019, Vol. 30 
