b School of Pharmacuetical Sciences, Guizhou Medical University, Guiyang 550025, China;
c Key Laboratory of Chemistry for Natural Products of Guizhou Province, Chinese Academy of Sciences, Guiyang 550014, China;
d Key Laboratory of Marine Drugs, Ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, China
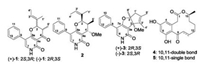
Endophyte is a class of microorganisms refers to the fungi and bacteria which invade or live inside the tissues of plants without causing any noticeable symptoms [1]. Endophytic fungi could enhance the ecological adaptability for their hosts and protect them from infections by pathogenic microorganisms [2, 3]. In our continuous investigations of bioactive natural products from endophytic fungi [4-7], strain GZWMJZ-313 identified as Penicillium sumatrense was isolated from the leaf of G. multiflora. A chemical study on its secondary metabolites led to the identification of three new pyridine alkaloids, citridones E–G (1–3), along with two curvularins (4 and 5) (Fig. 1).
|
Download:
|
| Fig. 1. The structures of compounds 1–5. | |
The strain in this work was isolated from the leaf of Garcinia multiflora, collected in September 2014, in Libo, Guizhou Province of China, which were identified by Dr. Wei Gu. A voucher specimen (H20140909) was deposited at the Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences. Fungal identification was carried out using ITS phylogenetic tree analyses (Fig. S28 in Supporting information), which identified the fungal strain GZWMJZ-313 as Penicillium sumatrense. ITS gene sequence data were deposited in NCBI GenBank, assigned with accession number MH259811. BLAST searching result showed that sequence has 99% sequence identity to that of Penicillium sumatrense CBS 416.69 (NCBI GenBank accession No. AY213677). A reference culture is stored in our laboratory of Guizhou at -80 ℃. The fungal strain was cultured on potato dextrose agar (PDA) at 28 ℃ for 3 days to prepare the seed culture. Plugs of agar supporting mycelium growth were cut and transferred aseptically to 20 × 1000 mL Erlenmeyer flasks each containing rice medium composed of 100 g rice and 120 mL distilled water. The flask was incubated at room temperature under static conditions for 30 days. The cultures were extracted three times by ethyl acetate (EtOAc) (500 mL for each) and the combined EtOAc extracts were dried in vacuo to yield 12.8 g of extract.
The EtOAc extract (12.8 g) was chromatographed on a silica gel column using step gradient elution of CH2Cl2–MeOH (0–100%, v/v) to yield seven fractions (1–7). Fraction 5 (0.6 g) was subjected to a silica gel column, elution with petroleum ether–EtOAc (3:1, v/v) to afford two subfractions. Subfraction 5–2 (82 mg) was purified by semi-preparative HPLC (60% MeOH/H2O) to yield 4 (35.6 mg, tR 7.1 min). Fraction 7 (4.3 g) was subjected to a silica gel column, eluted with CH2Cl2–MeOH (10:1, v/v) to afford 5 (1.2 g) and six subfractions. Fraction 7-6 (0.9 g) was further separated into two subfractions by Sephadex LH-20 eluting with MeOH-CH2Cl2 (1:1, v/v). Subfraction 7-6-2 (158 mg) was purified by semi-preparative HPLC (80% MeOH/H2O) to yield racemic 3 (9.3 mg, tR 8.2 min), 2 (8.2 mg, tR 8.9 min) and two subfractions. Subfraction 7-6-2-2 (46 mg) was purified by semi-preparative HPLC (65% MeOH/H2O) to yield racemic 1 (11.3 mg, tR 16.3 min). The racemic 1 was further purified over a Phenomenex LUX Cellulose-2 column (90% MeOH/ H2O) to give the optically-pure (+)-1 (0.7 mg, tR 14.2 min) and (–)-1 (0.9 mg, tR 18.3 min) (Fig. S25A in Supporting information). The racemic 3 was further purified over a Phenomenex LUX Cellulose-2 column (90% MeOH/H2O) to give the optically-pure (+)-3 (0.5 mg, tR 8.3 min) and (–)-3 (0.5 mg, tR 9.6 min) (Fig. S25D in Supporting information). Optically-pure 2 showed one peak both in chiral HPLC analyses using Cellulose-2 and Cellulose-5 column (Figs. S25B and C in Supporting information).
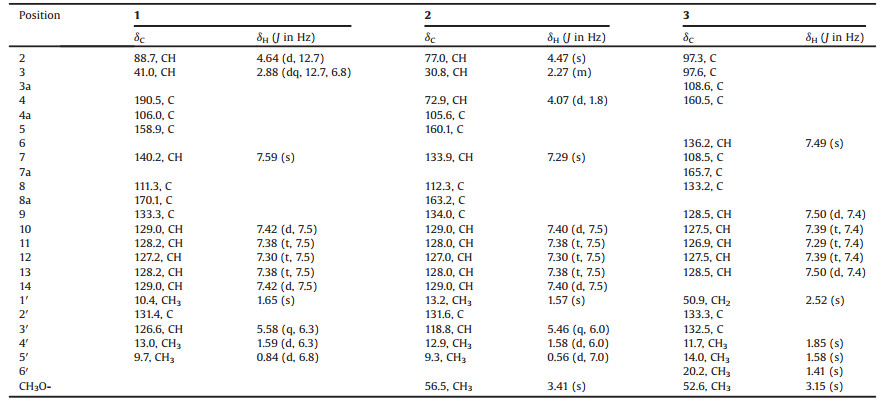
Citridone E (1) was isolated as colorless needle crystal, with a molecular formula of C19H19NO3 as deduced from the HRESIMS at m/z 310.1446 [M+H]+ (Calcd. for C19H20NO3: 310.1438) (Fig. S8 in Supporting information), indicating 11 degrees of unsaturation. The 1D and HMQC NMR spectra (Table 1, Figs. S1–S4 in Supporting information) displayed seven sp2-quaternary carbon signals (including two carbonyl carbons), seven sp2-methines signals, two sp3-methine signals (including an oxygenated one) and three methyl signals. The 1H-1H COSY (Fig. 2 and Fig. S5 in Supporting information) cross-peaks of H-10 to H-14 revealed the presence of a mono-substituted benzene ring. The HMBC (Fig. 2 and Fig. S6 in Supporting information) correlations from H-7 (δH 7.59) to C-8a (δC 170.1)/C-5 (δC 158.9)/C-9 (δC 133.3), H-10/14 (δH 7.42) to C-8 (δC 111.3) and H-11/13 (δH 7.38) to C-9 revealed the presence of a 5- phenylpyridone unit. These data showed good agreement with those of citridone A and CJ-16, 170 [8, 9]. The 1H-1H COSY crosspeaks of H-2 (δH 4.64) to H3-5' (δH 0.84) through H-3 (δH 2.88), and H-3' (δH 5.58) to H3-4' (δH 1.59) along with the HMBC correlations from H-2 to C-4 (δC 190.5)/C-8a/C-1' (δC 10.4)/C-3' (δC 126.6), H3-1' (δH 1.65) to C-3', H3-4' to C-2' (δC 131.4), H3-5' to C-4 revealed the presence of 2-(but-2-en-2 yl)-3-methyl-pyrone unit. These evidences assigned the planar structure of 1 as displayed in Fig. 1. The large 3JH-2,H-3 value (12.7 Hz) and ROESY correlation of H-2 to H-50 (Fig. 2 and Fig. S7 in Supporting information) revealed 2, 3-trans configuration. The relative configuration of compound 1 was further affirmed by the X-ray diffraction analysis (Fig. 3). The space group P-1 [10] in the X-ray diffraction and the line of the electronic circular dichroism (ECD) spectrum indicated 1 as a racemate. Fortunately, with the help of a Phenomenex LUX Cellulose-2 column, the optically pure (+)-1 and (–)-1 were resolved (Fig. S25 A in Supporting information). The absolute configuration of (+)-1 and (–)-1 were assigned as (2S, 3R)- and (2R, 3S)-, respectively, by comparing the ECD with those calculated using the time dependent density functional theory (TD-DFT) at the B3LYP/6– 31 G(d) level [11, 12] (Figs. S26 A and S27 A in Supporting information).
|
|
Table 1 1H (400 MHz) and 13C (100 MHz) NMR data of compounds 1–3 in DMSO-d6. |

|
Download:
|
Fig. 2. Key HMBC   |
|

|
Download:
|
| Fig. 3. X-ray crystallographic structure of (±)-1. | |
Citridone F (2) was isolated as a white powder, with a molecular formula of C20H23NO3 as deduced from the HRESIMS m/z 326.1757 [M+H]+ (Calcd. for C20H24NO3: 326.1751) (Fig. S16 in Supporting information), indicating 10 degrees of unsaturation. The 1D and HMQC NMR spectra (Table 1, Figs. S9–S12 in Supporting information) displayed six sp2-quaternary carbons' signals (including one carbonyl carbon), seven sp2-methines signals, three sp3-methine signals (including two oxygenated methines) and four methyl signals (including one methoxy group). These data were very close to those of compound 1, indicating the same molecular skeleton. The major differences were the absence of the carbonyl signal at C-4 and the presence of a methoxy substituted methine signals at δH/δC 3.41/56.5 and 4.07/72.9. In addition, C-2 and C-3 were obviously shifted to upfield, indicating that the C-4 carbonyl was changed to the oxygenated methine. The 1H-1H COSY (Fig. S13 in Supporting information) from H-2 (δH 4.47) through H-3 (δH 2.27) to H-4 (δH 4.07) and HMBC (Fig. S14 in Supporting information) correlation from the methoxy proton (δH 3.41) to C-4 (δC 72.9) further indicated this replacement. The ROESY cross-peaks (Fig. 2 and Fig. S15 in Supporting information) of H-2/H-3/methoxy proton, H-4/H3-5' (δH 0.56)/H3-1' (δH 1.57) revealed 2, 3-cis and 3, 4-trans configurations, which was further supported by the small coupling constants of H-2 with H-3 and H- 3 with H-4 [6]. The ROESY cross-peaks (Fig. 3 and Fig. S15) of H3-1'/ H1-3' (δH 5.46) disclosed the Z-Δ2'-double bond. The absolute configuration of 2 was resolved by the measurement and calculation of ECD. The calculated ECD of (2R, 3R, 4S)-2 and (2S, 3S, 4R)-2 were obtained by means of the TD-DFT at the B3LYP/6–31 G(d) level [11, 12]. The measured ECD is consistent with the calculated ECD of (2R, 3R, 4S)-2 but opposite that of (2S, 3S, 4R)-2 (Figs. S26B and S27B in Supporting information). Thus, the (2R, 3R, 4S)- configuration was assigned to optically-pure compound 2 that was supported by one peak in chiral HPLC analysis with both Cellulose-2 and Cellulose-5 columns (Figs. S25B and S25C in Supporting information).
Citridone G (3) was isolated as a white powder, with the molecular formula of C20H21NO3 from the HRESIMS peak at m/z 324.1598 [M+H]+ (Calcd. for C20H22NO3: 324.1594) (Fig. S24 in Supporting information), indicating 11 degrees of unsaturation. The 1D and HMQC NMR spectra (Table 1, Fig. S17–S20 in Supporting information) revealed seven sp2-quaternary carbons (including one carbonyl), six sp2-methines, two sp3-quaternary carbons (including an oxygenated one), a sp3-methylene and four methyls (including one methoxy). These data were quite similar to those of the known citridone A [9], except that the Δ1'-double bond shifted to Δ2' and an additional methoxy was placed at C-3. This was further supported by the HMBC correlations (Fig. 2 and Fig. S22 in Supporting information) from methoxy proton (δH 3.15) to C-3 (δC 97.6), H3-4' (δH 1.85) to C-3/C-2' (δC 133.3) and H3-5' (δH 1.58) to C-1' (δC 50.9)/C-3' (δC 132.5). In the ROESY spectrum (Fig. 2 and Fig. S23 in Supporting information), cross peak between H3-6' (δH 1.41) and methoxy proton suggested cis-orientation between 2- CH3 and 3-OCH3. The line of the ECD spectrum showed that compound 3 was racemic. Fortunately, with the help of a Phenomenex LUX Cellulose-2 column, the optically pure (+)-3 and (–)-3 were resolved (Fig. S25D in Supporting information). The absolute configuration of (+)-3 and (–)-3 were respectively assigned as (2R, 3S)- and (2S, 3R)- by comparing the experimental ECD with those calculated using TD-DFT at the B3LYP/6–31 G(d) level [11, 12] (Figs. S26C and S27C in Supporting information).
The two known compounds were identified as (–)-dehydrocurvularin (4) [13] and (–)-curvularin (5) [14], by comparison of their NMR spectra (Table S1 in Supporting information) and specific rotation with those reported in the literature. The [α]D20 values of 4 and 5 were –30.0 (c 2.0, MeOH) and –64.0 (c 1.0, MeOH), respectively.
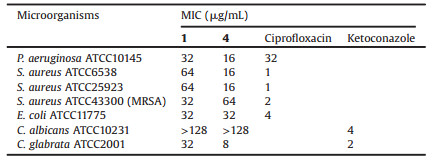
All the isolates were evaluated for their inhibitory effects on seven pathogens. Compounds 1 and 4 showed antibacterial and antifungal activities against Pseudomonas aeruginosa, Staphylococcus aureus, Clostridium perfringens, Escherichia coli and Candida albicans with MIC values from 8 μg/mL to 64 μg/mL (Table 2).
|
|
Table 2 Antimicrobial activities of compounds 1 and 4. |
In summary, we identified three new pyridine alkaloids, citridones E–G (1–3) produced by the endophytic fungus Penicillium sumatrense GZWMJZ-313. Citridone E (1) showed antimicrobial activities. And an unusual phenomenon that racemic and optically pure phenylpyridone derivatives from the same fungus was reported.
AcknowledgmentsThis research was financially supported by the 100 Leading Talents of Guizhou Province (fund for W. Zhu and X. Hao), the Science and Technology Project of Guizhou (No. QKHT Z-2014- 4007) and the Academician Workstation of Guizhou (No. QKH YSZ- 2015-4009).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.08.015.
| [1] |
G. Strobel, B. Daisy, U. Castillo, P. Harper, J. Nat. Prod. 67 (2004) 257-268. DOI:10.1021/np030397v |
| [2] |
R.X. Tan, W.X. Zou, Nat. Prod. Rep. 18 (2001) 448-459. DOI:10.1039/b100918o |
| [3] |
H. Hussain, N. Root, F. Jabeen, et al., Chin. Chem. Lett. 25 (2014) 1577-1579. DOI:10.1016/j.cclet.2014.06.006 |
| [4] |
Y. Li, K.L. Sun, Y. Wang, et al., Chin. Chem. Lett. 24 (2013) 1049-1052. DOI:10.1016/j.cclet.2013.07.028 |
| [5] |
Z.H. Xin, W.M. Zhu, Q.Q. Gu, et al., Chin. Chem. Lett. 16 (2005) 1227-1229. |
| [6] |
L.P. Wang, X.L. Han, G.L. Zhu, et al., Front. Chem 6 (2018) 344. DOI:10.3389/fchem.2018.00344 |
| [7] |
K.L. Sun, G.L. Zhu, J.J. Hao, Y. Wang, W.M. Zhu, Tetrahedron 74 (2018) 83-87. DOI:10.1016/j.tet.2017.11.039 |
| [8] |
S. Sakemi, J. Bordner, D.L. Decosta, et al., J. Antibiot. 55 (2002) 6-18. DOI:10.7164/antibiotics.55.6 |
| [9] |
T. Fukuda, H. Tomoda, S. Omura, J. Antibiot. 58 (2005) 315-321. DOI:10.1038/ja.2005.39 |
| [10] |
D.S. Tian, P. Yi, L. Xia, et al., Org. Lett. 18 (2016) 5904-5907. DOI:10.1021/acs.orglett.6b03004 |
| [11] |
N. Berova, L.D. Bari, G. Pescitelli, Chem. Soc. Rev. 36 (2007) 914-931. DOI:10.1039/b515476f |
| [12] |
Z.B. Chen, J.J. Hao, L.P. Wang, et al., Sci. Rep. 6 (2016) 20004. DOI:10.1038/srep20004 |
| [13] |
B.P. Bashyal, E.M.K. Wijeratne, J. Tillotson, et al., J. Nat. Prod. 80 (2007) 427-433. |
| [14] |
P.M. Tadross, S.C. Virgil, B.M. Stoltz, Org. Lett. 12 (2010) 1612-1614. DOI:10.1021/ol100335y |
 2019, Vol. 30
2019, Vol. 30