Polyacetylenes, characterized by possessing conjugated carbon-carbon triple bonds in structural skeleton, are a kind of secondary metabolites distributed widely in organisms, comprising plants [1], marine organisms [2, 3], microorganisms [4], and animals [5]. This class of compounds has aroused great interest of medicinal chemists and pharmaceutical industries due to its broad variety of biological properties, such as anti-inflammatory [6], cytotoxic [7, 8], and immunosuppressive [9] activities. During our ongoing program aimed at searching for bioactive constituents from Notopterygium incisum, a traditional Chinese herb used for treatment of inflammation-related diseases [10], a sub-fraction of its 95% aq. EtOH was found to exhibit potent cytotoxicity against different cancer cells. Subsequent chemical investigation led to the isolation of 14 polyacetylenes, including a new compound, notopolyenol A (1) (Fig. 1).

|
Download:
|
| Fig. 1. Structures of compounds 1–14. | |
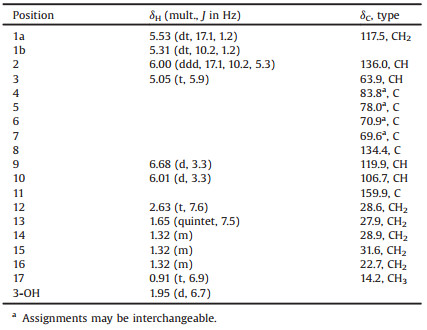
Notopolyenol A (1) was isolated as a colorless oil with the molecular formula of C17H20O2 determined by HREIMS at m/z 256.1456 [M+] (calcd. for C17H20O2: 256.1458), corresponding to eight indices of hydrogen deficiency. The 1H NMR spectrum (Table 1) displayed characteristic signals for a terminal vinyl group [δH 6.00 (ddd, 1H, J = 17.1, 10.2, 5.3 Hz), 5.53 (dt, 1H, J = 17.1, 1.2 Hz), and 5.31 (dt, 1H, J = 10.2, 1.2 Hz)], an aliphatic chain [δH 2.63 (t, 2H, J = 7.6 Hz), 1.65 (quintet, 2H, J = 7.5 Hz), 1.32 (m, 6 H), and 0.91 (t, 3H, J = 6.9 Hz)], and two coupled furan protons [δH 6.68 (d, 1H, J = 3.3 Hz) and 6.01 (d, 1H, J = 3.3 Hz)]. Inspection of the 13C NMR data (Table 1) in combination with the HSQC correlations classified 17 carbons into two conjugated acetylenic bonds (δC 83.8, 78.0, 70.9, and 69.6), two trisubstituted double bonds (δC 134.4, 119.9 and 159.9, 106.7), a monosubstituted double bond (δC 117.5 and 136.0), an oxygenated sp3 methine (δC 63.9), five sp3 methenes (δC 31.6, 28.9, 28.6, 27.9, and 22.7), and one methyl (δC 14.2). The aforementioned information suggested that 1 was a derivative of falcarindiol (5) [11]with thepresenceof afuran ring consistedof C-8 (δC134.4), C-9 (δH6.68, δC119.9), C-10 (δH6.01, δC106.7), anδC-11 (δC 159.9), which accounted for the one remaining index of hydrogen deficiency. The HMBC correlations of H-9/C-8 and C-11, H-10/C-8 and C-11, and H2-12/C-10 and C-11 and the NOESY correlation of H- 10/H2-12 (Fig. S3 in Supporting information) supported the above deduction. Thus, the 2D structure of 1 was defined as shown.
|
|
Table 1 1H (400 MHz) and 13C (100 MHz) NMR data of compound 1 (δ in ppm) in CDCl3. |
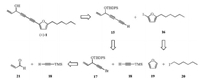
To establish the absolute configuration of 1, the modified Mosher's method was carried out. However, attempts to prepare two Mosher's esters of 1 failed due to the limitation of its amount (1.1 mg). Therefore, the total synthesis of racemic (±)-1 should be conducted, and its retrosynthetic analysis is depicted in Scheme 1. (±)-1 could be constructed by Sonogashira reaction [12] from diyne 15 and iodofuran 16. Disconnection at the conjugated acetylenic bond of 15 would give two alkynes 17 and 18 as intermediates for Cadiot-Chodkiewicz coupling [13], while the former subunit could be further disassembled into aldehyde 21 and TM(S)-protected acetylene 18 as substrates of 1, 2-addition. Fragment 16 would be prepared by substitution reaction from furan 19 and alkyl iodide 20.
|
Download:
|
| Scheme 1. Retrosynthetic analysis of compound (±)-1. | |
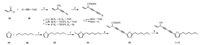
The synthesis of (±)-1 is summarized in Scheme 2. 1, 2-Addition of acrolein (21) with trimethylsilylacetylene (18) in the presence of n-BuLi followed by protection of the resulting hydroxyl in 22 with t-butyldiphenylsilyl (TBDPS) group afforded 23 as a silyl ether. Treatment of 23 with N-bromosuccinic imide (NBS) and catalytic amount of AgNO3 gave brominated alkyne 17, which was coupled with 18 subsequently via Cadiot-Chodkiewicz reaction [13] to furnish the conjugated diyne 24. The terminal TMS group of 24 was then selectively removed in K2CO3/MeOH to obtain 15. The coupling reaction of 15 with 16, which was prepared by alkylation of furan (19) with 1-iodohexane (20) using n-BuLi followed by iodination, under Sonogashira reaction condition [12] yielded 26 smoothly. Finally, deprotection of the TBDPS group of 26 by tetran-butylammonium fluoride (TBFA) provided (±)-1 in 20.6% overall yield for 7 steps from 21.
|
Download:
|
| Scheme 2. Synthesis of compound (±)-1. Reagents and conditions: (a) n-BuLi, THF, –78 ℃ to r.t., 87% for 22, 70% for 25; (b) TBDPSCl, Et3N, DMAP, δCM, 0 ℃ to r.t., 90%; (c) NBS, AgNO3, Me2CO, r.t. 88%; (d) 18, CuCl, n-BuNH2, NH2OH·HCl, δCM, 0 ℃, 63%; (e) K2CO3, MeOH, r.t., 82%; (f) I2, n-BuLi, THF, –78 ℃ to 0 ℃, 70%; (g) 15, CuI, PPh3, (PPh3)Pd2Cl2, Et3N, 60 ℃, 69%; (h) TBAF, DCM, r.t., 84%. | |
HPLC chiral resolution of (±)-1 afforded two enantiomers, (-)-1 at 8.7 min and (+)-1 at 11.4 min, respectively. Both enantiomers were subjected to esterification with (R)- and (S)-α-methoxyphenylacetic acid (MPA) to obtain the corresponding esters, and analyses of their ΔδRS (δR –δS) values led to the assignment of 3R configuration for (-)-1 and 3S configuration for (+)-1 (Fig. S1 in Supporting information). By comparison of the HPLC chromatogram and ECD cruve of 1 with those of the synthetic products (Figs. S4 and S5 in Supporting information), the absolute configuration of 1 was unambiguously defined as 3R.
A plausible biogenetic pathway to 1 is proposed with falcarindiol (5), a known co-isolated compound with 3R and 8S configuration determined by the modified Mosher's method (Fig. S2 in Supporting information), as the precursor (Scheme S1 in Supporting information). Allylic oxidation of 5 resulted in the generation of carbonyl group at C-11 followed by nucleophilically attacked by hydroxyl group at C-8. Then the removal of H2O induced the electrons transfer and formation of a furan ring, and finally converted to 1.
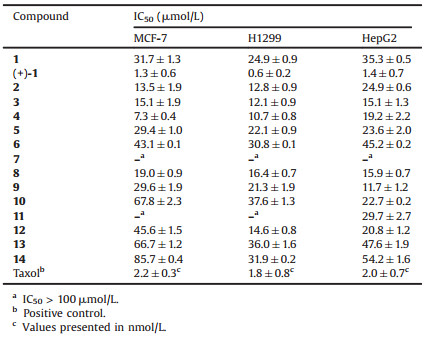
All isolated compounds 1-14, as well as the synthetic product (+)-1, were evaluated for their cytotoxicity against three cancer cell lines, MCF-7, H1299, and HepG2, using the sulforhodamine B (SRB) assay [14], and their IC50 values are presented in Table 2. Of these, the synthetic compound (+)-1 exhibited the most potent cytotoxic activity against the three cancer cell lines with IC50 values ranging from 0.6 μmol/L to 1.4 μmol/L, at least 24-fold lower than those of its enantiomer 1, indicating the importance of 3S configuration for the cytotoxic effect. Panaxydiol-type polyacetylenes (2–4), with IC50 values of 10.7–24.9 μmol/L, displayed stronger inhibitory effects on the test cancer cells than those of most of falcarindioltype polyacetylenes (5–12) and their reduction products (13 and 14), suggesting that the conjugated system enlarged by 8E-double bond may play a positive role in their cytotoxicity.
|
|
Table 2 Cytotoxic effects of compounds 1–14 and (+)-1 against three cancer cell lines. |
In summary, a new polyacetylene (1), together with thirteen analogues (2–14), was isolated from the roots and rhizomes of N. incisum. Its absolute configuration was determined as 3R by applying the modified Mosher's method to synthetic enantiomers (-)-1 and (+)-1 followed by comparing their HPLC retention times and ECD spectra. Interestingly, the synthetic product (+)-1, the enantiomer of 1, displayed the most significant cytotoxic activity against three cancer cell lines (MCF-7, H1299, and HepG2).
AcknowledgmentThis wok was financially supported by the National Key Technology R & D Program "New Drug Innovation" of China (No. 2018ZX09711001-008-003).
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.09.011.
| [1] |
K. Xu, Z. Feng, J. Jiang, et al., Chin. Chem. Lett. 28 (2017) 597-601. DOI:10.1016/j.cclet.2016.10.036 |
| [2] |
M. Kladi, C. Vagias, P. Papazafiri, et al., J. Nat. Prod. 72 (2009) 190-193. DOI:10.1021/np800481w |
| [3] |
E.J. Mejia, L.B. Magranet, N.J. de Voogd, et al., J. Nat. Prod. 76 (2013) 425-432. DOI:10.1021/np3008446 |
| [4] |
J. Chen, W. Lin, C. Liao, et al., J. Nat. Prod. 70 (2007) 989-992. DOI:10.1021/np070045e |
| [5] |
G. Fabrias, M. Barrot, F. Camps, Insect Biochem. Molec. Biol. 25 (1995) 655-660. DOI:10.1016/0965-1748(95)00003-E |
| [6] |
M.C. Yang, H.C. Kwon, Y. Kim, et al., J. Nat. Prod. 73 (2010) 801-805. DOI:10.1021/np900628j |
| [7] |
S. Sun, G. Du, L. Qi, et al., J. Ethnopharmacol. 132 (2010) 280-285. DOI:10.1016/j.jep.2010.08.026 |
| [8] |
H.R. Jin, J. Zhao, Z. Zhang, et al., Cell Death Dis. 3 (2012) e376. DOI:10.1038/cddis.2012.122 |
| [9] |
S. Mitsui, K. Torii, H. Fukui, et al., J. Pharmacol. Exp. Ther. 333 (2010) 954-960. DOI:10.1124/jpet.109.162305 |
| [10] |
Editorial Committee of Chinese Materia Medica of State Administration of Traditional Chinese Medicine, Chinese Materia Medica, Shanghai Scientific & Technical Publishers, Shanghai, 1999, p. 992.
|
| [11] |
D. Lechner, M. Stavri, M. Oluwatuyi, et al., Phytochemistry 65 (2004) 331-335. DOI:10.1016/j.phytochem.2003.11.010 |
| [12] |
K. Ma, Y. Miao, X. Gao, et al., Chin. Chem. Lett. 28 (2017) 1035-1038. DOI:10.1016/j.cclet.2016.11.032 |
| [13] |
B.V. Subba Reddy, R. Nageshwar Rao, B. Kumaraswamy, J.S. Yadav, Tetrahedron Lett. 55 (2014) 4590-4592. DOI:10.1016/j.tetlet.2014.06.054 |
| [14] |
V. Vichai, K. Kirtikara, Nat. Protoc. 1 (2006) 1112-1116. DOI:10.1038/nprot.2006.179 |
 2019, Vol. 30
2019, Vol. 30