b Institute of Drug Synthesis and Pharmaceutical Process, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, China
Vinylsulfonium salts are a class of special Michael acceptors with diversified reactivities. They had been used as efficient ethylene transfer reagents for the synthesis of various valuable products. Their reactions with amino aldehydes or ketones generated heterocyclic epoxides [1]. They were also used to react with bisheteroatomic nucleophiles for the preparation of heterocyclic compounds [2]. The reaction of vinylsulfonium salts with active methylene compounds or primary amines provided cyclopropanes and aziridines [3]. In addition, 1-butadienyl-sulfonium salts were applied for the synthesis of epoxides via the conjugate addition and subsequent intramolecular cyclization [4]. Huang and co-workers explored the applications of allenic sulfonium salts for the synthesis of fused heterocycles [5]. Recently, we developed an annulation reaction of vinylsulfonium salts with β-naphthols and 1, 4-hydroxycoumarins. A series of dihydrofuran derivatives were prepared in moderate to good yields [6]. Despite these progresses, only few functionalized vinylsulfonium salts were prepared and applied to new synthetic reactions [1f, 3b,3c]. Their reactivities and synthetic potentials remain to be explored. As a continuous effort to explore the new synthetic applications of vinylsulfonium salts, herein we report the first synthesis of β-alkoxycarbonyl vinylsulfonium salts and their application for the cyclopropanation of active methylene compounds. A variety of cyclopropane carboxylates could be prepared in good yields.
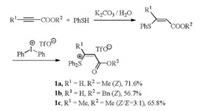
The synthesis of β-alkoxycarbonyl vinylsulfonium salts 1a-1c is showed in Scheme 1. The conjugate addition of thiophenol to propiolates provided 3-(phenylthio) acrylates in good yields [7]. The further phenylation with diphenyliodonium triflate gave β- alkyloxycarbonyl vinylsulfonium salts 1a-1c [8]. Only Z-isomers were detected for 1a-1b, but a small amount of E-isomer was observed for 1c. The experiment details please see Supporting information.
|
Download:
|
| Scheme 1. Synthesis of β-alkoxycarbonyl vinylsulfonium salts 1a-1c. | |
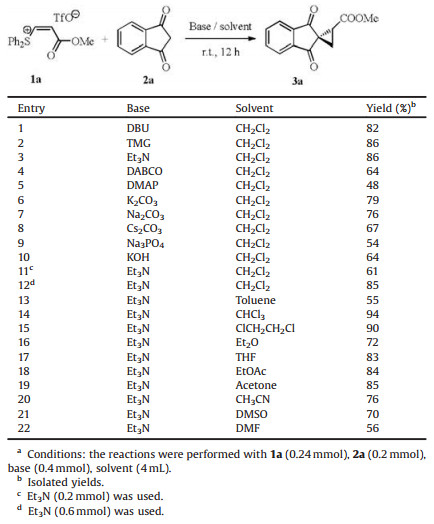
The reaction of 1a with 1, 3-indenedione 2a was examined and the results are summarized in Table 1. The reaction underwent smoothly in the presence of organic bases DBU (1, 5-diazabicyclo- [4.3.0]non-5-ene), TMG (tetramethylguanidine) and Et3N, but lower yields were obtained with DABCO (1, 4-diazabicyclo[2. 2. 2] octane) and DMAP ((4-dimethylamino-pyridine)(Table 1, entries 1-5). Inorganic bases are also efficient (Table 1, entries 6-10). Considering the yield and convenient work-up, Et3N was selected for the further study. The optimal amount of Et3N was identified as 2 equiv. More or less amount of Et3N led to decreased yields (Table 1, entries 11 and 12). The effect of reaction solvent was also examined (Table 1, entries 13-22). The reaction occurred in various organic solvents. The best yield was achieved in CHCl3.
|
|
Table 1 Optimization of reaction conditions.a |
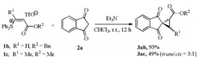
The reactions of 1b and 1c with 2a were also examined (Scheme 2). The good reactivity was observed for 1b, but 1c is less reactive probably due to the increased steric hindrance. The product 3ac was obtained in 49% yield as a mixture of trans/cis isomers.
|
Download:
|
| Scheme 2. The reactions of 1b or 1c with 2a. Conditions:1b/1c (0.24 mmol), 2a (0.2 mmol), Et3N (0.4 mmol), and CHCl3(4 mL). Isolated yields. | |
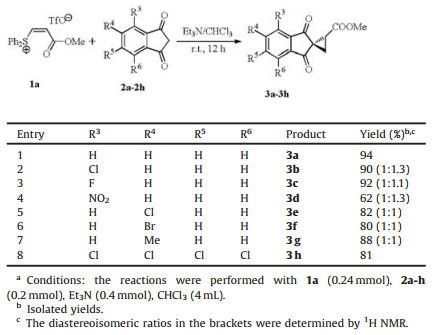
The reaction of a series of 1, 3-indene-diones 2a-h with 1a were examined and the results are summarized in Table 2. The substitutions on the benzene ring with 4-Cl and 4-F were tolerated well. Excellent yields were obtained for the products 3b-3c. The 4- NO2 substituted 2d afforded a lower yield. The substitutions with 5-Cl, 5-Br and 5-Me led to slightly lower yields. Due to the different direction of the ester group with 4-or 5-substituent, products 3b-g consisted of two diastereoisomers. The ratios were determined by 1H NMR spectra. The reaction of 4, 5, 6, 7-tetrachcloro-1, 3-indenedione 2h provided the product 3h in a good yield.
|
|
Table 2 The reaction of 1, 3-indene-diones 2a-h with 1a.a |
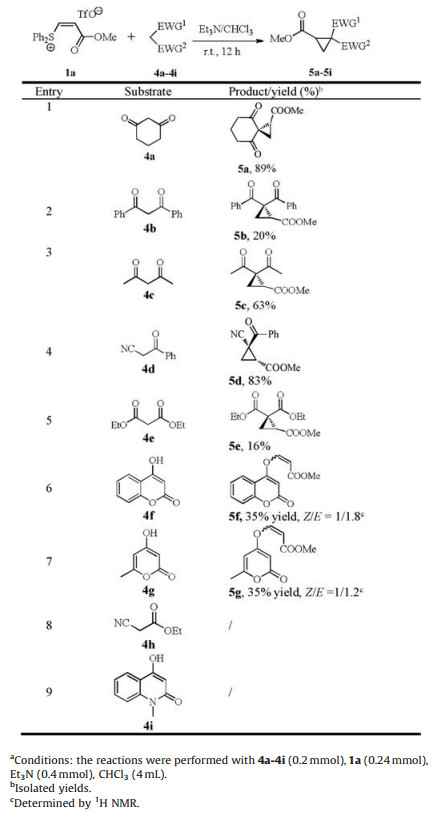
Furthermore, the reactions of 1a with a series of active methylene compounds 4a-g were examined and the results are summarized in Table 3. 1, 3-Cyclohexanedione 4a afforded the expected product 5a in an excellent yield. 1, 3-Diphenylpropane- 1, 3-dione 4b showed low reactivity. The reaction of acetoacetone 4c provided the product 5c in a moderate yield. α-Cyano acetophenone 4d showed good reactivity. The product 5d was obtained in an excellent yield. The reaction of diethyl malonate 4e is not satisfactory. The product 5e was obtained in a poor yield. On the other hand, the reaction of coumarin 4f and its analogue 4g did not afford the expected products, instead O-vinyl products 5f and 5g were obtained in low yields. Ethyl α-cyanoacetate 4h and the substrate 4i were found to be unreactive (For the details of synthetic procedures, see Supporting information).
|
|
Table 3 The reaction of 1a with active methylene compounds 4a-i.a |
A tentative reaction mechanism was proposed (Scheme 3). The deprotonation of 1, 3-indenedione 2a with Et3N gives the enolate anion. The conjugate addition of the anion to 1a generates the sulfur ylide A. After the second deprotonation of 1, 3-indenedione, the intermediate B is formed. Subsequent cyclization provides the product 3a and eliminates diphenylsulfide.

|
Download:
|
| Scheme 3. Proposed reaction mechanism. | |
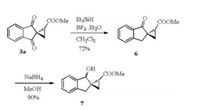
The reduction of the product 3a was studied (Scheme 4). The reaction with NaBH4 afforded the complicated stereoisomers of the alcohols. The treatment with Et3SiH gave selectively the monodecarbonyl product 6 [9]. The ester group obviously shielded the carbonyl group on the same side. Only the carbonyl group on the opposite was reduced. The further reduction of 6 with NaBH4 provided the alcohol 7 as a mixture of two diastereoisomers (details in Supporting information).
|
Download:
|
| Scheme 4. Reduction of the product 3a. | |
We have developed an efficient synthetic route of β-alkoxycarbonyl vinylsulfonium salts. Their reactions with indene-1, 3- diones and other active methylene compounds were studied. A series of cyclopropane carboxylates were prepared in good yields. The characterization data of all products are posited in Supporting information. A tentative reaction mechanism was proposed. Further applications of β-alkoxycarbonyl vinylsulfonium salts to new synthetic reactions are currently underway.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21772240, 21472248), National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2017ZX09305010), Guangzhou Science Technology and Innovation Commission (No. 201707010210), Department of Science and Technology of Guangdong Province (Nos. 2017A020211011, 2017A020211027) and China Scholarship Council (No. 201608440090) for the financial support of this study.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.08.021.
| [1] |
(a) Y. F. Wang,W. H. Zhang, V. J. Colandrea, L. S. Jimenez, Tetrahedron 55 (1999) 10659-10672; (b) Z. Wang, L. S. Jimenez, J. Am. Chem. Soc. 116 (1994) 4977-4978; (c) K. H. Kim, L. S. Jimenez, Tetrahedron Asymmetry 12 (2001) 999-1005; (d) M. G. Unthank, N. Hussain, V. K. Aggarwal, Angew. Chem. Int. Ed. 45 (2006) 7066-7069; (e) M. G. Unthank, B. Tavassoli, V. K. Aggarwal, Org. Lett. 10 (2008) 1501-1504; (f) S. P. Fritz, T. H. West, E. M. McGarrigle, V. K. Aggarwal, Org. Lett. 14 (2012) 6370-6373. |
| [2] |
(a) M.Yar, E. M. McGarrigle, V. K. Aggarwal, Angew. Chem. Int. Ed. 47 (2008) 3784-3786; (b) M. Yar, E. M. McGarrigle, V. K. Aggarwal, Org. Lett. 11 (2009) 257-260; (c) E. M. McGarrigle, S. P. Fritz, L. Favereau, M. Yar, V. K. Aggarwal, Org. Lett. 13 (2011) 3060-3063; (d) J. An, N. J. Chang, L. D. Song, et al., Chem. Commun. 47 (2011) 1869-1871; (e) C. S. Xie, D. Y. Han, Y. Hu, J. H. Liu, T. Xie, Tetrahedron Lett. 51 (2010) 5238-5241. |
| [3] |
(a) H. Braun, G. Huber, Tetrahedron Lett. 17 (1976) 2121-2124; (b) K. Takaki, T. Agawa, J. Org. Chem. 42 (1977) 3303-3304; (c) R. Maeda, K. Oyama, R. Anno, et al., Org. Lett. 12 (2010) 2548-2550; (d) H. Lin, Q. L. Shen, L. Lu, J. Org. Chem. 76 (2011) 7359-7369. |
| [4] |
(a) M. E. Garst, J. Org. Chem. 44 (1979) 1578-1580; (b) M. E. Garst, P. Arrhenius, J. Org. Chem. 48 (1983) 16-24; (c) M. W. Rowbottom, N. Mathewsb, T. Gallagher, J. Chem. Soc. Perkin Trans. I 1 (1998) 3927-3930. |
| [5] |
(a) P. H. Jia, Q. L. Zhang, Q. M. Ou, Y. Huang, Org. Lett. 19 (2017) 4664-4667; (b) P. H. Jia, Q. L. Zhang, H. X. Jin, Y. Huang, Org. Lett. 19 (2017) 412-415. |
| [6] |
Z.C. Chen, L. Tong, Z.B. Du, et al., Org. Biomol. Chem. 16 (2018) 2634-2638. DOI:10.1039/C8OB00293B |
| [7] |
Y.M. Li, C. Mgck-Lichtenfeld, A. Studer, Angew. Chem. Int. Ed. 55 (2016) 14435-14438. DOI:10.1002/anie.v55.46 |
| [8] |
T. Ishikawa, N. Kasai, Y. Yamada, T. Hanamoto, Tetrahedron Lett. 17 (2015) 1254-1260. |
| [9] |
Y.Y. Liu, X.Y. Yu, J.R. Chen, et al., Angew. Chem. Int. Ed. 56 (2017) 9527-9531. DOI:10.1002/anie.v56.32 |
 2019, Vol. 30
2019, Vol. 30