b Liaoning University of Traditional Chinese Medicine-Agilent Technologies Modern TCM and Multi-Omics Research Collaboration Lab, Liaoning University of Traditional Chinese Medicine, Dalian 116600, China
Activity, toxicityand compatibilityare the threekey itemsin early drug discovery and development. How to predict the appropriate concentration and compatibility proportions with maximum efficacy and minimal toxicity of compounds for a system is critical for drug discovery. Previous studies were typically pursued in traditional screening methods, including animal experiment in vivo (whole animal level, tissue and organ level) and cell experiment in vitro, all of these took an important place in the research and development of new drugs [1-3]. However, there are still some disadvantages, such as the high cost and difficult manipulation of animal models, defective physical simulation environment of human body by cell models cultivation in 96-well plate, which give rise to the problems of long R & D cycle, large input cost and low success rate [4-6], and etc. Therefore, a fast, low-cost and sensitive detection method is essential for drug discovery and development.
In the wake of developments in science and technology, the progress of genomics, proteomics and microfluidic technology have promoted drug screening and compatibility research. Microfluidic technology is attracting growing interest in recent years. It is based on the science that deals with the fabrication of microdevices with small channels and chambers, control of the flow behavior of small volume of fluids in micro-channels and microchambers, whose dimensions are in the range of tens to hundreds of micrometers [7-9]. Microfluidic techniques enable a continuous nutrient and oxygen supply, allow the integration of multiple steps such as cell culture, cell capture, cell lysis, mixing and enabling the modelling of physiological conditions more accurately on the same device [10-12]. Microfluidic technologies have many advantages, such as reducing the consumption of reagents samples to picoliters, decreasing the reaction time to seconds, reducing waste generation [13, 14], etc. All of these can achieve high-throughput, rapid activity, toxicity and compatibility screening of drug. With the development of material science and life science, the microfluidic chips are using new materials as the carrier, and their functions are becoming more and more abundant. For example, organ chip that mimics the special structure of a human organ and the functional chips focused on enrichment, screening and detection have been successfully developed. These chips with different functions greatly increase the research efficiency, shorten the research cycle and reduce the research cost. Applications in the field of drug research, especially in the field of Chinese medicine, are increasing rapidly. In recent years, using the microfluidic chip as carrier, the models of cell transport and tumor angiogenesis in vivo were successfully made, and rapid detection was realized, and drug screening was successfully carried out at the cell level.
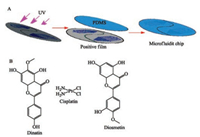
In this paper, we described a multifunctional integrated microfluidic biochip that could cultivate disease cells and healthy cells on the same chip for detecting the efficacy-toxicity and compatibility of medicine. The chip was designed based on the theory of "Yin-Yang" in traditional Chinese Taiji. In the theory of traditional Chinese philosophy, Yin symbolizes the negativity, cold and darkness and Yang symbolizes the positive, heat and light. Yin and Yang are two opposing principles in nature. Coincidentally, the efficacy and toxicity of drug are also unity of opposites. In present study, the concept was incorporated into drug study. Yang symbolizes the inhibit effect of drugs on cancer cells, and Yin symbolizes the toxicity of drugs to normal cells. In our chip, the right (Yang) and left part (Yin) could be injected into different kinds of cells for online detecting activity and toxicity simultaneously through the centralized control. Meanwhile, the microchannel of right (Yang) and left part (Yin) was design based on the structure of the Christmas tree. At the top of the structure, there were two liquid entrances. After the two kinds of liquid mixed in the chip, then the mixed liquid would be diluted in the hierarchical structure. In this way, a set of 8 concentrations could be generated at the same time. In addition, if both liquid entrances passed through the same concentration of solution, we could also centrally detect the efficacy of a single concentration. The main structure of this biochip consisted of PDMS, the positive film (silicon mould) was developed by Corel DRAW Graphics Suite X7, and the photoresist was solidified on the silicon wafer using the soft lithography technology, thus formed the positive film with the fixed shape. To cure the PDMS pre-polymer, a mixture containing the silicone elastomer and the curing agent (10:1 wt ratio) was poured onto the silicon mould and baked at 95℃ for 40 min. Then the bonding PDMS pre-polymer and cleaned glass sheet were bond using a plasma cleaning machine [15-18]. The design and manufacture diagram of this biochip is shown in Figs. 1 and 2.
|
Download:
|
| Fig. 1. (A) The design and manufacture diagram of microfluidic chip. (B) Chemical structure formula of dinatin, cisplatin and diosmetin. | |

|
Download:
|
| Fig. 2. Schematic diagram of chip. | |
To verify the function of the chip, we selected cisplatin (chemotherapy drug, has renal toxicity), dinatin and diosmetin (natural products of Traditional Chinese Medicine from the total flavonoids of Cirsium setosum (Wild. ) which their anticancer activity has been reported in the literature [19, 20]. ) as the research object to carry out the efficacy-toxicity and compatibility of these three drugs (Fig. 1B). The applicability of the chip was clarified by comparing the results obtained by the biochip with the results obtained by the 96-well plate. The cells were seeded into the microfluidic cell channel at a density of 5×106 cells/mL and perfusion-cultured at a flow rate of 0.03 mL/h for 24 h. We used the Hoechst 33342 and PI to stain the cells in multiple channel of the chip. Inverted fluorescence microscope was used to photograph the multiple channels at specific wave lengths. Then IPP (Image-Pro Plus) software was used to calculate the rate of cell apoptosis and necrosis rate by the collection of pixels. In general, stronger the fluorescence was, there would be more apoptotic and necrotic cells, indicate that the IC50 values of the inhibition rate of dinatin, diosmetin and cisplatin were 0.04 mg/mL, 0.05 mg/mL and 5 μL/mL respectively. It is worth mentioning that we used cisplatin injection to configure the required concentration, so the concentration of cisplatin was 5 μL/mL. Correspondingly, the apoptosis and necrosis rate of HEK293 cells was 31.76%, 26.21% and 48.67%. To reflect the toxicity of the drug, the survival rate (100%- inhibition ratio) of HEK293 cells were used as the evaluation criterion. The calculated survival rate of dinatin, diosmetin and cisplatin was 68.24%, 73.79%, 51.39%. At the same concentration, the survival rate of the three drugs on HEK293 cells was 69.56%, 71.49% and 49.06% in the 96-well plates. Then, results of chip and 96-well plate were imported into SPSS software for Pearson correlation analysis. The results showed there was a positive correlation between the two groups of data. In evidence, there was no significant difference between the results of biochip and 96- well flat-bottomed plate, indicating the chip has high accuracy on activity-toxicity detecting.
After determining the activity-toxicity of each compound, a total of 9 different compatibilities of three compounds were conducted using uniform design method based on IC50 dosage as the upper limit. The 9 experimental results are shown in Table 1. According to the above test method, the efficacy and toxicity of different compatibility groups were detected. Significantly, we found different group has different inhibition ratio to A549 cell and different toxicity on HEK293 cells and we can infer that appropriate dose compatibility may reduce the toxicity of cisplatin and increase inhibitory effect on A549 cells. Our results of the chip indicated that when the concentrations of the three drugs are 0.0375 mg/mL (dinatin), 0.05 mg/mL (diosmetin) and 1.25 μL/mL (cisplatin) in the compatibility group 1, the inhibition rate is the highest and the toxicity is the least. The inhibitory rate on A549 cells was 65.72% and the survival rate of HEK293 cells is 84.44%. The results of 96-well plates showed the fraction surviving of compatibility Group 1 on HEK293 cells was 79.72% and inhibitory rate on A549 cells was 63.29%. We also imported the results of chip and 96-well plate into SPSS software for Pearson correlation analysis. The results showed that there was a positive correlation between the two groups of data. There was no significant difference between the results of biochip and 96-well flatbottomed plate, indicating that the chip has high accuracy (compatibility results were shown in Fig. 3B).
|
|
Table 1 Drug concentration in each compatibility group. |

|
Download:
|
| Fig. 3. (A) The calculated survival rate of cisplatin, dinatin and diosmetin to HEK293 cells. (B) The results of each compatibility groups. | |
As a high-throughput screening method, microfluidic chip was centralized controlled by precision injection pump. The microfluidic chip was perfused dynamically in millimeter chip aperture at the bottom velocity, which could better simulate the environment in vivo and be closer to the physiological condition of cells. Over 24 h, total culture medium was less than 0.5 mL, which greatly reduced the consumption of test drugs. This chip offers many advantages, such as high-throughput, rapid and closely to the real environment of human body, be applicable for activity, toxicity and compatibility screening of drug.
In summary, a microfluidic biochip for efficacy-toxicity and compatibility testing was successfully manufactured and proved to be multifunctional. This microfluidic biochip could detect the efficacy-toxicity of medicine simultaneously, as well as detect the best compatibility of medicine with maximum efficacy and minimal toxicity. Using the chemotherapeutics to cure cancer has been clinical application for many years. However, the large side effects, especially the toxicity to liver and kidney have been restricting the development of chemotherapeutic drugs. In this paper, we use the microfluidic chip and uniform design method to compatibility of two active natural extracts with cisplatin. Increased the inhibitory effect of compounds on tumor cells and reduced damage to normal renal cells.
Hence, it provides a potentially interesting tool to predict toxicology, screen pharmaceutical drug and explore drug mechanism, also offer a new idea for the combination of tumor therapy, chemotherapeutic drugs and active substances of traditional Chinese medicine. The development of this chip has the vital significance to the research of new drugs.
Due to the space constraints, some of the substances cannot be elaborated in detail. So we put the details of the experiment in the Supporting inforamtion for your reference.
Appendix A.Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.08.016.
| [1] |
J. Tao, J. Xu, F. Chen, et al., Eur. J. Pharm. Sci. 111 (2018) 540-548. DOI:10.1016/j.ejps.2017.10.039 |
| [2] |
Y. Ma, Y. Bao, S. Wang, et al., Inflammation 39 (2016) 1453-1461. DOI:10.1007/s10753-016-0377-4 |
| [3] |
Y. Wang, Z. Sun, M. Szyf, Oncotarget 8 (2017) 111866-111881. |
| [4] |
J.M. McKim Jr, H. Baas, G.P. Rice, et al., Food Chem. Toxicol. 96 (2016) 1-10. DOI:10.1016/j.fct.2016.07.006 |
| [5] |
A. Falanga, M. Marchetti, Thromb. Res. 164 (2018) S54-S61. DOI:10.1016/j.thromres.2018.01.017 |
| [6] |
M.T. Abreu, P. Vora, E. Faure, et al., J. Immunol. 167 (2001) 1609-1616. DOI:10.4049/jimmunol.167.3.1609 |
| [7] |
N.C.A. van Engeland, A.M.A.O. Pollet, J.M.J. den Toonder, et al., Lab Chip 18 (2018) 1607-1620. DOI:10.1039/C8LC00286J |
| [8] |
H. Zhang, L. Xiao, Q. Li, et al., Biomicrofluidics 12 (2018) 024119-024124. DOI:10.1063/1.5024359 |
| [9] |
M. Oshiki, T. Miura, S. Kazama, et al., Front. Microbiol. 27 (2018) 830. |
| [10] |
C.P. Miller, C. Tsuchida, Y. Zheng, et al., Neoplasia 20 (2018) 610-620. DOI:10.1016/j.neo.2018.02.011 |
| [11] |
S.N. Bhatia, D.E. Ingber, Nat. Biotechnol. 32 (2014) 760-772. DOI:10.1038/nbt.2989 |
| [12] |
Y. Liu, X. Jiang, Lab Chip 17 (2017) 3960-3978. DOI:10.1039/C7LC00627F |
| [13] |
J. Fan, Y. Bao, X. Meng, et al., Oncotarget 19 (2017) 31395-31405. |
| [14] |
J. Chamoun, A. Pattekar, F. Afshinmanesh, et al., Lab Chip 18 (2018) 1581-1592. DOI:10.1039/C7LC01266G |
| [15] |
F. Zarghampour, Y. Yamini, M. Baharfar, M. Faraji, J. Chromatogr. A 1556 (2018) 21-28. DOI:10.1016/j.chroma.2018.04.046 |
| [16] |
A. Bein, W. Shin, S.J. Firoozinezhad, et al., Cell. Mol. Gastroenterol. Hepatol. 5 (2018) 659-668. DOI:10.1016/j.jcmgh.2017.12.010 |
| [17] |
S. Han, J. Kim, R. Li, et al., Adv. Healthc. Materr. 7 (2018) 1800122. DOI:10.1002/adhm.v7.12 |
| [18] |
Y. Zhang, Z. Wang, L. Wu, et al., Small 14 (2018) 1704433-1704436. DOI:10.1002/smll.v14.20 |
| [19] |
X. Li, X. Ying, H. Yan, et al., Chin. Pharm. J. 10 (2017) 1624-1628. |
| [20] |
C. Song, B. Shen, S. Zhu, et al., J. Clin. Emergency 4 (2017). |
 2019, Vol. 30
2019, Vol. 30