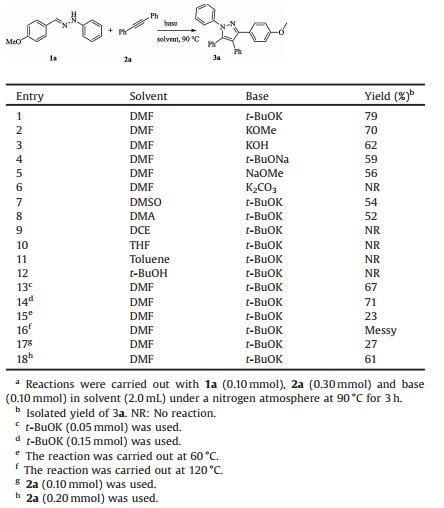
Multisubstituted pyrazole structural motifs are frequently found in many natural products, pharmaceuticals, agrochemicals, functional materials, and ligands for transition-metal catalysis [1]. For example, biologically active compounds such as anti-inflammatory celecoxib [2], anticancer obinutuzumab [3], anorectic antiobesity rimonabant [4], and insecticidal fipronil [5] contain these structural motifs (Fig. 1). As a result, extensive efforts have been made to develop practical and efficient methods for the synthesis of multisubstituted pyrazoles [6, 7]. The developed conventional methods include:1) condensation of 1, 3-diketones or α, β-unsaturated carbonyl compounds with substituted hydrazines, 2) condensation of hydrazines or tetrazoles with alkynes, and 3) multiple arylations of unsubstituted pyrazoles. Despite the significant progresses have been made, most methods are restricted by the requirement of transition metal catalyst, insufficient regioselectivity and low availability of substrates. Thus, developing new methods to meet the purpose of synthetic divergence, especially under metal-free conditions, is highly desirable.
|
Download:
|
| Fig. 1. Biologically active pyrazoles. | |
Metal-free radical reactions have attracted much attention from chemists in the past decades. Some elegant work via hydrazone radicals to construct various molecules has been studied. In 2013, Chiba and co-workers reported an aliphatic C-H bond oxidation of hydrazones by TEMPO to prepare substituted pyrazoles [7d]. The hydrazone radical was considered as a key species in the transformation. In the same year, Han demonstrated the synthesis of pyrazolines and tetrahydropyridazines via hydrazone radicals formed by the similar protocol [8]. However, the reactions of hydrazone radicals with alkynes to form pyrazoles have never been reported. In recent years, we have developed a series of t-BuOK/ DMF promoted radical reactions of tertiary amines and amides with alkenes, alkynes and ketones [9]. It was found that t-BuOK/ DMF could promote the formation of nitrogen-centered radicals including benzohydrazide and amidyl radicals [10]. We speculated that a hydrazone radical might be generated in t-BuOK/DMF system and then led to the subsequent transformations to synthesize nitrogen heterocycles. Herein, we report our discovery that a t-BuOK/DMF system could promote the cycloaddition of hydrazones and diarylalkynes. A series of tetraarylpyrazoles were obtained in moderate to good yields [11].
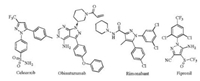
We initially examined the reaction of hydrazone 1a with diphenylethyne 2a in the presence of t-BuOK/DMF. To our delight, the desired product 3a was obtained in good yield. The reaction conditions were further optimized and the results are summarized in Table 1. Other bases such as KOMe, KOH, t-BuONa and NaOMe were examined under the similar conditions and were less effective (entries 2-5). When K2CO3 was utilized, the reaction did not work at all (entry 6) [9, 10]. Solvents affected the reactions very much. The reaction in DMSO and DMA gave 3a in moderate yields (entries 7-8). Other solvents such as DCE, THF, toluene and t-BuOH are incompatible (entries 9-12) [9b]. The loading of t-BuOK was also examined. Increase or decrease the load of t-BuOK resulted in slight loss of yield (entries 13 and 14). The reactions at 60 ℃ and 120 ℃ were also examined and the lower yields were obtained. The reaction at 60℃ was not complete and some starting material 1a was recovered. At 120℃, complicated products were observed and no pure product 3a could be isolated. The amount of 2a also affected on the yield of 3a. Lower yields were obtained while 1 or 2 equiv. of 2a was applied (entries 17 and 18). Some unreacted 1a was observed in these cases by TLC analysis. The complete consume of 1a was achieved while 3 equiv. of 2a were used. A part of 2a may be decomposed under the strongly basic condition, so excess amount of 2a is required for a better yield.
|
|
Table 1 Optimization of the reaction conditions. a |
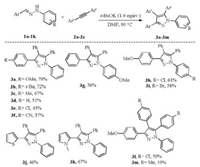
With the optimized reaction conditions in hand, a number of hydrazones 1a-1k were examined under the similar conditions and the results are summarized in Fig. 2. The electron-donating substitutions (OMe, t-Bu and Me) on 1-benzylidene moiety provided good yields (3a-3c). In contrast, the electron-withdrawing substitutions such as Cl and CN exerted detrimental effect on the yields (3e and 3f). The effect of the substitutions on the phenyl group of the nitrogen of hydrazones was also explored. Electrondonating and-withdrawing groups had a slight impact on the yield (3g-3i). It is worthy to note that heteroaryl hydrazones (1j-1k) were successfully converted to the desired products under the conditions. The corresponding products 3j-3k were obtained in moderate yields. This cycloaddition reaction is sensitive to alkynes. When 1, 2-bis (4-chlorophenyl) ethyne 2b was used as the substrate, the desired product 3l was obtained in 59% yield. Moreover, when 1, 2-bis (4-methylphenyl) ethyne 2c was utilized for the reaction, the yield of product was poor. 1, 2-Bis (4-(trifluoromethyl) phenyl) ethyne did not work. This result is in consistent with our previous observations that trifluoromethyl substitution generally inhibits the radical reaction in t-BuOK/DMF system [9]. Furthermore, 1, 2- bis (4-methoxyphenyl) ethyne, hex-3-yne, 4-(phenylethynyl) benzonitrile and phenylacetylene gave messy products.
|
Download:
|
| Fig. 2. Cyclization of hydrazones 1a-1k with 1, 2-diarylalkynes 2a-2c. Reaction conditions:1(0. 10mmol), 2(0. 30 mmol) and t-BuOK (0. 10mmol) in DMF (2. 0 mL) under a nitrogen atmosphere at 90℃ for 3h. | |
To explore the reaction mechanism, radical trapping experiments were performed (Scheme 1). The reaction was completely inhibited in the presence of DPPH (1, 1-diphenyl-2-icrylhydrazyl radical), benzoquinone or oxygen. The yield was reduced to 20% when TEMPO (2, 2, 6, 6-tetramethylpiperidinooxy) was added. These results are in accordance with a free radical pathway.

|
Download:
|
| Scheme 1. Control experiments with free radical scavengers. | |
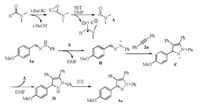
Based on the control experiments and our previous studies [9a], a possible reaction mechanism is proposed (Scheme 2). Although the anion pathway cannot be excluded completely, it is less reasonable than the radical pathway [12]. The carbamoyl radical A generated in t-BuOK/DMF system abstracts a hydrogen atom from hydrazone 1a. The resulting hydrazone radical B undergoes a cycloaddition with 1, 2-diphenylethylene 2a to give the intermediate C. A hydrogen transfer from DMF or another molecule 1a to intermediate C provides dihydropyrazole D as the intermediate. Further oxidation gave the final product 3a. The electron-donating groups on 1-benzylidene moiety favor the formation of free radical intermediate B. This is why 3a was obtained in better yields than other products.
|
Download:
|
| Scheme 2. Proposed reaction mechanism. | |
In conclusion, we have developed a metal-free, simple and practical synthetic approach for the synthesis of tetraarylpyrazoles by the cycloaddition reaction of hydrazone and 1, 2-diphenylethyne promoted by t-BuOK/DMF. The desired products were obtained in moderate to good yields. A possible reactions mechanism through hydrazone radical is proposed. This work provides a new strategy for the synthesis of tetrarylpyrazoles in one step.
AcknowledgmentsWe thank the National Natural Science Foundation of China(No. 21472248), Department of Science and Technology of Guangdong Province(No. 2017A020211011) for the financial support of this study.
Appendix A.Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.06.018.
| [1] |
(a) A. Çetin, Int. J. Curr. Res. Chem. Pharma. Sci. 2 (2015) 8-20; (b)M. F. Khan, M. M. Alam, G. Verma, et al., Eur. J. Med. Chem. 120 (2016) 170-201; (c)X. L. Deng, J. Xie, Y. Q. Li, et al., Chin. Chem. Lett. 27 (2016) 566-570; (d)A. Ansari, A. Ali, M. Asif, New J. Chem. 41 (2017) 16-41; (e)A. S. Hassan, M. F. Mady, H. M. Awad, T. S. Hafez, Chin. Chem. Lett. 28 (2017) 388-393. |
| [2] |
P.L. McCormack, Drugs 74 (2014) 2457-2489. |
| [3] |
F. Cameron, P.L. McCormack, Drugs 74 (2014) 147-154. DOI:10.1007/s40265-013-0167-3 |
| [4] |
T. Yoshioka, T. Fujita, T. Kanai, et al., J. Med. Chem. 32 (1989) 421-428. DOI:10.1021/jm00122a022 |
| [5] |
D. Hainzl, J.E. Casida, Proc. Natl. Acad. Sci. U. S. A. 93 (1996) 12764-12767. DOI:10.1073/pnas.93.23.12764 |
| [6] |
(a) S. Maddila, S. B. Jonnalagadda, K. K. Gangu, S. N. Maddila, Curr. Org. Synth. 14 (2017) 634-653; (b)S. Fustero, M. Sánchez-Roselló, P. Barrio, A. Simón-Fuentes, Chem. Rev. 111 (2011) 6984-7034. |
| [7] |
(a) S. Fuse, T. Morita, K. Johmoto, H. Uekusa, H. Tanaka, Chem. Eur. J. 21 (2015) 14370-14375; (b)M. Ye, A. J. F. Edmunds, J. A. Morris, et al., Chem. Sci. 4 (2013) 2374-2379; (c)X. Zhu, Y. F. Wang, W. Ren, F. L. Zhang, S. Chiba, Org. Lett. 15 (2013) 3214-3217; (d)X. Li, L. He, H. Chen, W. Wu, H. Jiang, J. Org. Chem. 78 (2013) 3636-3646; (e)M. A. Sanchez-Carmona, D. A. Contreras-Cruz, L. D. Miranda, Org. Biomol. Chem. 9 (2011) 6506-6508; (f)J. J. Shi, G. H. Ren, N. J. Wu, et al., Chin. Chem. Lett. 28 (2017) 1727-1730. |
| [8] |
X.Y. Duan, X.L. Yang, R. Fang, et al., J. Org. Chem. 78 (2013) 10692-10704. DOI:10.1021/jo4016908 |
| [9] |
(a) Y. Y. Chen, X. J. Zhang, H. M. Yuan, W. T. Wei, M. Yan, Chem. Commun. 49 (2013) 10974-10976; (b)W. T. Wei, X. J. Dong, S. Z. Nie, et al., Org. Lett. 15 (2013) 6018-6021; (c)W. J. Wang, X. Zhao, L. Tong, et al., J. Org. Chem. 79 (2014) 8557-8565; (d)W. T. Wei, Y. Liu, L. M. Ye, et al., Org. Biomol. Chem. 13 (2015) 817-824; (e)Y. Y. Chen, J. H. Chen, N. N. Zhang, et al., Tetrahedron Lett. 56 (2015) 478-481. |
| [10] |
(a) W. J. Wang, J. H. Chen, Z. C. Chen, et al., Synthesis 48 (2016) 3551-3558; (b)Z. Y. Chen, L. Y. Wu, H. S. Fang, et al., Adv. Synth. Catal. 357 (2017) 3894-3899. |
| [11] |
S Mukherjee, P.S. Salini, A. Srinivasan, S. Peruncheralathan, Chem.Commun. 51 (2015) 17148-17151. DOI:10.1039/C5CC05973A |
| [12] |
(a) C. L. Øpstad, T. B. Melø, H. R. Sliwka, V. Partali, Tetrahedron 65 (2009) 7616-7619; (b)M. P. Drapeau, I. Fabre, L. Grimaud, et al., Angew. Chem. Int. Ed. 54 (2015) 10587-10591. |
 2019, Vol. 30
2019, Vol. 30