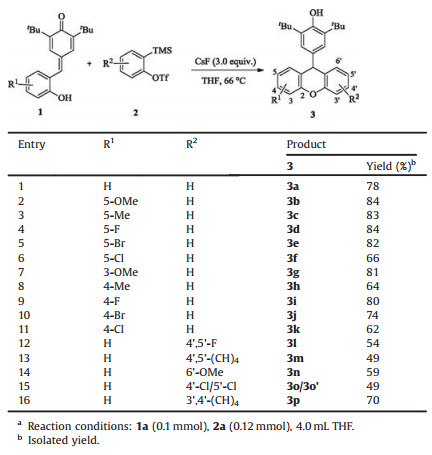
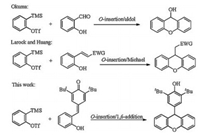
Xanthenes are significant architectural motif found in biologically active compounds and pharmaceuticals [1]. In particular, some xanthene derivatives can be used as electronic sensors [2], fluorescent dyes [3] and photocatalysts [4]. Therefore, the synthesis and applications of these scaffolds have received tremendous interest in the past several decades. The conventional methods for the synthesis of xanthenes typically involve the Friedel-Crafts reaction to construct the central ring. In general, many of these approach suffering some drawbacks, such as harsh reaction conditions, multistep reactions and complicated procedure, which limit their practical application. Benzyne [5] is highly reactive intermediate that utilized broadly in the synthesis of heterocycles. Recently, through the cascade O-insertion-aldol reaction of salicyaldehyde and benzynes, Okuma and co-workers developed an interesting method for the synthesis of 9-hydroxy xanthenes [6]. Following this excellent work, O-insertion-Michael addition reaction between ortho-hydroxy substituted Michael acceptors and benzynes were further developed by Larock [7] and Huang [8], providing 9-functionalized xanthenes in a single step (Scheme 1).
|
Download:
|
| Scheme 1. Insertion-cycloaddition reactions of benzyne. | |
para-Quinone methides (p-QMs) [9] are versatile building blocks in organic synthesis. Owing to their thermodynamic driving force of rearomatizations, p-QMs can undergo 1, 6-conjugate addition with different nucleophiles to form diarylmethine compounds [10]. Recently, for the first time, using the ortho-hydroxyphenyl substituted p-QMs as a donor-acceptor, Enders [11] and co-workers developed an excellent asymmetric oxa-michael/ 1, 6-conjugate addition cascade reaction. As part of our ongoing studies on benzyne chemistry [12], we envisaged that the ortho-hydroxyphenyl substituted p-QMs can react with benzyne via a cascade insertion/1, 6-conjugate addition reaction to form 9- phenol substituted xanthenes. During the preparation of this paper, a similar research was reported by Shi and co-workers [13]. And herein, we wish to report our independent results.
Our initial studies were carried out with the readily available benzyne precursor 2a [14] and p-QMs 1a as test substrate. With 2.4 equiv. KF/18-crown-6 as additives, benzyne smoothly reacted with p-QMs to produce the described xanthene 3a in 30% yield. At the same time, the O-phenylation product 4a was isolated as the major side product in 41% yield (Table 1, entry1). Interestingly, when CsF/ 18-crown-6 were used instead of KF/18-crown-6, xanthene 3a was obtained in 44% yield as the major product (Table 1, entry 2). However, using 2.4 equiv. CsF as the sole additive, no desired product was obtained (Table 1 entry 3). Other fluoride sources, such as TBAF and TMAF cannot mediate the cascade reaction. TBAT promoted the reaction in moderate conversion, but with lower chemselectivity (Table 1, entry 6). The following simple screening of the reaction media revealed that THF is the best choice with respect to the yield and selectivity (Table 1, entries 2, 7–9). Elevating the reaction temperature cannot improve the reaction yield and selectivity (Table 1, entries 10–12). Interestingly, with 2.4 equiv. CsF as the sole additive and conducted the reaction in refluxing THF led to improved yield and chemselectivity (Table 1, entry 13). Pleasingly, higher yield can be obtained when the reaction proceeded in diluted solution (Table 1, entry 14). However, further decreasing the concentration of the substrates led to the reduction of the yield and selectivity (Table 1, entry 15). Increasing the amount of CsF to 3.0 equiv. resulted in higher yield and selectivity (Table 1, entry 16). However, prolonged the reaction time to 24 h or further increased the amount of additive to 4.0 equiv. had no obvious effects on the reaction (Table 1, entries 17 and 18).
|
|
Table 1 Optimization of reaction conditions.a |
With the optimal reaction conditions in hand (Table 1, entry 16), we next studied the scope and generality of the cascade addition. As shown in Table 2, different substituted ortho-hydroxyphenyl derived p-QMs reacted with benzyne smoothly to deliver the desired xanthenes in high yields (Table 2, entries 1–6). Moreover, the electronic properties and different positions of the substituents have no obvious effects on the reaction (Table 2, entries 7-11). Other symmetric benzynes, such as 4, 5-difluro-substitutied benzyne and β, β-naphthalene, coupled with p-QMs 1a to furnish the corresponding products 3 l and 3 m in 54% and 49% yield, respectively (Table 2, entries 12 and 13). In accordance with many other cycloadditions, the asymmetric 3-methoxybenzyne underwent the reaction in complete regioselectivity (Table 2, entry 14). However, when asymmetric benzyne precursor 2e was applied for the reaction, a 1:1 regioisomers were obtained in 49% yield as an inseparable mixture (Table 2, entry 15). Notably, the unsymmetrical α, β-naphthalene provided the desired product 3p in 70% yield with excellent regioselectivity (Table 2, entry 16).
|
|
Table 2 Evaluation of substrate scope.a |
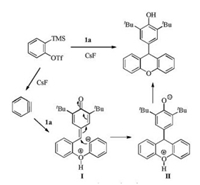
Based on the pioneering studies of the insertion-cycloaddition of benzyne, a plausible mechanism was proposed as depicted in Scheme 2. The phenol hydroxyl group insert to benzyne to form zwitterion Ⅰ, which undergoes 1, 6-conjugate addition with p-QMs to form species Ⅱ, and after the following protonation, to provide the xanthene product.
|
Download:
|
| Scheme 2. Proposed mechanism. | |
In conclusion, the cascade O-phenylation/1, 6-conjugate addition reaction of benzyne has been described. The mild and transition-metal free condition, generally good to high yields and selectivity provide an efficient protocol for the synthesis of 9- phenol substituted xanthenes.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21462034), the Young Scientists Foundation of Shihezi University (Nos. RCZX201546, 2015ZRKXJQ05) and the Excellent Young Teachers Plan of Bingtuan (No. CZ027203).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.04.003.
| [1] |
(a) N. Sato, M. Jitsuoka, T. Shibata, et al., J. Med. Chem. 51 (2008) 4765-4770; (b) L. Äjemyr, T. Sandén, J. Widengren, P. Brzezinski, Biochemistry 48 (2009) 2173-2179; (c) M.C. de la Fuente, D. Dominguez, J. Org. Chem. 72 (2007) 8804-8810; (d) T. Kobayashi, Y. Urano, M. Kamiya, J. Am. Chem. Soc.129 (2007) 6696-6697. |
| [2] |
N. Fitchen, P. O'Shea, P. Williams, K.R. Hardie, Mol. Immunol. 40 (2003) 407-411. DOI:10.1016/S0161-5890(03)00153-6 |
| [3] |
(a) Y. Nagano, J. Am. Chem. Soc. 129 (2007) 10324-10325; (b) M.P. Okoh, J.L. Hunter, J.E.T. Corrie, M.R. Webb, Biochemistry 45 (2006) 14764-14771; (c) L. Äjemyr, T. Sanden, J. Widengren, P. Brzezinski, Biochemistry 48 (2009) 2173-2179. |
| [4] |
T. Lazarides, T. McCormick, P. Du, et al., J. Am. Chem. Soc. 131 (2009) 9192-9194. DOI:10.1021/ja903044n |
| [5] |
(a) S. Bhojgude, A. Bhunia, A.T. Biju, Acc. Chem. Res. 49 (2016) 1658-1670; (b) A. Bhunia, S.R. Yetra, A.T. Biju, Chem. Soc. Rev. 41 (2012) 3140-3152; (c) S. Bhojgude, A.T. Biju, Angew. Chem. Int. Ed. 51 (2012) 1520-1522; (d) P.M. Tadross, B.M. Stoltz, Chem. Rev. 112 (2012) 3550-3577; (e) A.V. Dubrovskiy, N.A. Markina, R.C. Larock, Org. Biomol. Chem. 11 (2013) 191-218; (f) C. Wu, F. Shi, Asian J. Org. Chem. 2 (2013) 116-125. |
| [6] |
K. Okuma, A. Nojima, N. Matsunaga, K. Shioji, Org. Lett. 11 (2009) 169-171. DOI:10.1021/ol802597x |
| [7] |
(a) J. Zhao, R.C. Larock, Org. Lett. 7 (2005) 4273-4275; (b) J. Zhao, R.C. Larock, J. Org. Chem. 72 (2007) 583-588; (c) C. Lu, A.V. Dubrovskiy, R.C. Larock, Tetrahedron Lett. 53 (2012) 2202-2205; (d) A.V. Dubrovskiy, R.C. Larock, Org. Lett. 12 (2010) 1180-1183; (e) Y. Fang, D.C. Rogness, R.C. Larock, F. Shi, J. Org. Chem. 77 (2012) 6262-6270. |
| [8] |
X. Huang, T. Zhang, J. Org. Chem. 75 (2010) 506-509. DOI:10.1021/jo902311a |
| [9] |
(a) P. Chauhan, U. Kaya, D. Enders, Adv. Synth. Catal. 359 (2017) 888-912; (b) M.M. Toteva, J.P. Richard, Adv. Phys. Org. Chem. 45 (2011) 39-91; (c) A. Parra, M. Tortosa, ChemCatChem 7 (2015) 1524-1526. |
| [10] |
(a) Z. Yuan, K. Gai, Y. Wu, et al., Chem. Commun. 53 (2017) 3485-3488; (b) Z. Yuan, L. Liu, R. Pan, H. Yao, A. Lin, J. Org. Chem. 82 (2017) 8743-8751; (c) Z. Yuan, R. Pan, H. Zhang, et al., Adv. Synth. Catal. 359 (2017) 4244-4249; (d) V. Reddy, R.V. Anand, Org. Lett. 17 (2015) 3390-3393; (e) P. Goswami, G. Singh, R.V. Anand, Org. Lett. 19 (2017) 1982-1985; (f) K. Zhao, Y. Zhi, A. Wang, D. Enders, ACS Catal. 6 (2016) 657-660; (g) Y. Lou, P. Cao, T. Jia, et al., Angew. Chem. Int. Ed. 54 (2015) 12134-12138; (h) W.D. Chu, L.F. Zhang, X. Bao, et al., Angew. Chem. Int. Ed. 52 (2013) 9229-9233; (i) X.Z. Zhang, Y.H. Deng, K.J. Gan, et al., Org. Lett. 19 (2017) 1752-1755; (j) Z. Wang, Y.F. Wong, J. Sun, Angew. Chem. Int. Ed. 54 (2015) 13711-13714; (k) Y.F. Wong, Z. Wang, J. Sun, Org. Biomol. Chem. 14 (2016) 5751-5754; (l) H. Lv, W. Jia, L. Sun, S. Ye, Angew. Chem. Int. Ed. 52 (2013) 8607-8610; (m) H. Lv, L. You, S. Ye, Adv. Synth. Catal. 351 (2009) 2822-2826; (n) J.J. Zhao, S.B. Sun, S.H. He, Q. Wu, F. Shi, Angew. Chem. Int. Ed. 54 (2015) 5460-5464; (o) J.J. Zhao, Y.C. Zhang, M.M. Xu, M. Tang, F. Shi, J. Org. Chem. 80 (2015) 10016-10024; (p) Y.C. Zhang, Q.N. Zhu, X. Yang, L.J. Zhou, F. Shi, J. Org. Chem. 81 (2016) 1681-1688; (q) P. Chen, K. Wang, W.G. Guo, et al., Angew. Chem. Int. Ed. 56 (2017) 3689-3693; (r) Z.B. Wang, J.W. Sun, Org. Lett. 19 (2017) 2334-2337. |
| [11] |
(a) K. Zhao, Y. Zhi, T. Shu, et al., Angew. Chem. Int. Ed. 55 (2016) 12104-12108; (b) S. Liu, X.C. Lan, K. Chen, et al., Org. Lett. 19 (2017) 3831-3834; (c) J.Y. Liao, Q. Ni, Y. Zhao, Org. Lett. 19 (2017) 4074-4077; (d) L. Liu, Z. Yuan, R. Pan, et al., Org. Chem. Front. 5 (2018) 623-628. |
| [12] |
(a) L. He, J.X. Pian, J.F. Shi, G.F. Du, B. Dai, Tetrahedron 70 (2014) 2400-2405; (b) J.X. Pian, L. He, G.F. Du, H. Guo, B. Dai, J. Org. Chem. 79 (2014) 5820-5826; (c) K. Liu, L.L. Liu, C.Z. Gu, B. Dai, L. He, RSC Adv. 6 (2016) 33606-33610; (d) L.L. Liu, Z.J. Li, C.Z. Gu, L. He, B. Dai, J. Saudi, Chem. Soc. 21 (2017) 458-465; (e) L. He, J.X. Pian, J.Y.Z. Zhang Li, Chin. Chem. Lett. 23 (2012) 1359-1362; (f) J. Zhang, K. Liu, H. Jian, Z.J. Li, L. He, Tetrahedron Lett. 58 (2017) 2964-2968; (g) H. Jian, K. Liu, W.H. Wang, et al., Tetrahedron Lett. 58 (2017) 1137-1141. |
| [13] |
G.J. Mei, S.L. Xu, W.Q. Zheng, C.Y. Bian, F. Shi, J. Org. Chem. 83 (2018) 1414-1421. DOI:10.1021/acs.joc.7b02942 |
| [14] |
Y. Himeshima, T. Sonoda, H. Kobayashi, Chem. Lett. 12 (1983) 1211-1214. DOI:10.1246/cl.1983.1211 |
 2019, Vol. 30
2019, Vol. 30