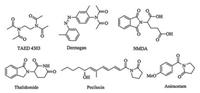
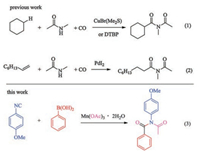
In nature, there are a wide range of compounds containing amide structures, which are important intermediates and structural units in organic synthesis [1]. In addition to amides, diimide structures are also important organic moieties in fine chemicals. For example, TAED, Dermagan, Formothion, Thalidomide, Pecilocin, etc. have properties such as insecticidal, anti-tumor, antifungal, and anti-anxiety drugs (Fig. 1) [2]. Therefore, it is of great significance to prepare chemical compounds with diimide structure. The traditional method for preparing diimides is achieved by nucleophilic substitution of an amide with an activated carboxylic acidderivative (such as an acid chloride or anhydride) in the presence of a base [3]. In addition, selective oxidation by amides is also known [4]. In recent years, the interest of researchers has been drawn to the use of carbon monoxide (CO) as the cheap C1 synthon to synthesize carbonyl-containing chemicals [5]. Recently, Prof. Wu's, Beller's, and Lei's group have reported the efficient use of CO for the synthesis of compounds containing imide structures (Scheme 1, Eqs. (1) and (2)) [6].
|
Download:
|
| Fig. 1. Examples of imide-containing bioactive molecules. | |
|
Download:
|
| Scheme 1. Synthesis of imide using CO along with this work. | |
Isonitriles are also important C1 synthons and play an important role in the synthesis of nitrogen-containing heterocyclic compounds [7, 8]. Free-radical reactions involving isonitriles are an important part of isonitrile chemistry. In recent years, the transition metal-catalyzed radical reactions involving isonitriles have been tremendously developed [9]. Our group has reported the transition metal-catalyzed diaryl isonitriles involved in free radical reactions to prepare phenanthridine derivatives [10]. Here, we first report the successful preparation of diimide derivatives by reaction of phenylboronic acid with isonitrile under the catalysis of Mn (OAc)3 (Scheme 1, Eq. (3)).
Initially, we attempted the reaction of 1-isocyano-4-methoxybenzene (1a), phenylboronic acid (2a), 3 equivalents of Mn(OAc)3·2H2O as oxidant in MeCN at 65℃ for 12 h, Gratifyingly, target product 3a could be obtained with 22% LC yield (Table 1, entry 1). The reaction failed to afford 3a when other oxidant, such as Cu(OAc)2 and AgOAc were used (Table 1, entries 2, 3). Then, we studied the effects of the solvent and found that 1, 4-dioxane, DMF, DCE, and DME did not give better results (Table 1, entries 4–7). Next we screened the reaction temperature and found that the reaction was best at 100℃, and the target product could be obtained with a 53% LC yield (Table 1, entries 8–10). Finally, we optimized the reaction time and found that the reaction yield was highest for 4 h (Table 1, entries 11–14). When we used a catalytic amount of Mn(OAc)3·2H2O and Mn(acac)3, 1.5 equiv. of NaOAc, the reaction failed to afford 3a (Table 1, entries 15, 16).
|
|
Table 1 Optimization of the reaction conditions.a |
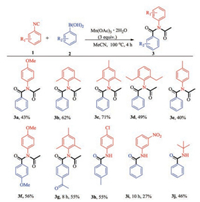
With the optimized conditions in hand, we began to study the scope of various aryl isonitriles (Scheme 2). It was found that when we used 2, 6-dimethyl aryl isocyanide, 2, 4, 6-trimethyl aryl isocyanide and 2, 6-diethyl aryl isonitrile to react with phenylboronic acid, the target products (3b–3d) could be obtained in 49%–71% yields. The reaction of 2a with 1-isocyano-4-methylbenzene 1e furnished the desired product 3e in 40% yield. The reaction of p-methoxyphenyl isonitrile with p-methoxyphenylboronic acid could result in the desired product 3f in 56% yield. Then we used 2, 4, 6-trimethylphenylisonitrile to react with p-acetylphenylboronic acid, the desired product 3 g was obtained in 55% yield. However, when we select the halogen-substituted and 3- nitro-substituted isonitriles to react with different substituted phenylboronic acids, The corresponding imide product was not obtained, but only the corresponding amide product (3h, 3i) was obtained. Besides, the reaction of tert-butylisonitrile with 2a also lead to the corresponding amide compounds 3j in 46% yield (detail information of 3a–j see Supporting information).
|
Download:
|
| Scheme 2. Scope study of isonitriles. Reaction conditions: 1 (0.2 mmol), 2 (0.24 mmol), Mn(OAc)3·2H2O (3.0 equiv.), MeCN (2 mL), under air. Isolated yield. | |
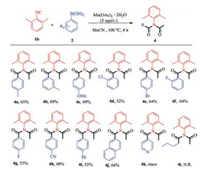
Thereafter, we investigated a range of different phenylboronic acids 2 (Scheme 3). When p-methylphenylboronic acid reacted with m-methylphenylboronic acid, the target product (4a, 4b) could be obtained in 65% and 69% yields, respectively. The reaction of p-methoxyphenylboronic acid and 1b afforded the desired product 4c in 49% yield. Then, when we selected halogensubstituted phenylboronic acid at different positions, the target product (4d–4g) could be obtained in 53%~64% yields. Electronwithdrawing groups, such as CN substituted phenylboronic acid (4h) could also lead to the desired products 4b in 40% yield. When [1, 1'-biphenyl]-4-ylboronic acid was subjected to the reaction with 1b, 4i could be obtained in 52% yield. The reaction of 2- naphthaleneboronic acid with 1b furnished the desired product (4j) in 64% yield. Unfortunately, the reactions of heterocyclefunctionalized boric acid 2k and alkylboronic acid 2l failed to give the desired products 4k and 4l (detail information of 4a–j see Supporting information).
|
Download:
|
| Scheme 3. Scope study of phenylboronic acids. Reaction conditions: 1 (0.2 mmol), 2 (0.24 mmol), Mn(OAc)3·2H2O (3.0 equiv.), MeCN (2 mL), under air. Isolated yield. | |
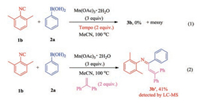
To gain insights into the mechanism of this reaction, two control experiments were conducted (Scheme 4). When 2 equiv. of TEMPO (2, 2, 6, 6-tetramethylpiperidine-1-oxyl) was added to the reaction system of 1b with 2a, As expected, the desired product N-acetyl-N-(2, 6-dimethylphenyl)benzamide (3b) was not detected, and the reaction system was very messy. This result indicated that the reaction may have experienced a free radical process. When 2 equiv. of another radical scavenger (1, 1-diphenylethylene) was added to the reaction system of 1b with 2a, We successfully obtained the captured imine radical (Ⅱ) product 3b' in an isolated yield of 41% (detail information of 3b' see Supporting information). It was proved that the reaction went through an imine free radical intermediate.
|
Download:
|
| Scheme 4. Control experiments. | |
Based on the literature reports [11] and the above experimental results, we proposed a plausible reaction mechanism in Scheme 5. First, the phenylboronic acid compound produce phenyl radical Ⅰ under the action of Mn(Ⅲ), then phenyl radical Ⅰ reacts with 1a to give the imine radical intermediate Ⅱ. Next, Ⅱ gives the nitrogen positive ion intermediate Ⅲ under the oxidation of Mn(Ⅲ) [12]. Ⅲ is attacked by acetate ions to obtain intermediate Ⅳ, which is then rearranged intramolecularly to give the target product diimide 3a.

|
Download:
|
| Scheme 5. A plausible mechanism. | |
In summary, we have reported a manganese(Ⅲ)-catalyzed oxidative radical cascade reaction of isonitrile and phenylboronic acid under mild conditions. A new C-C bond, C-N bond, and C=O bond were constructed in one step, and a series of diimide derivatives was successfully constructed. It provided a practical method for the preparation of diimide derivatives, which has the advantages of easy availability of starting materials, simple operation.
AcknowledgmentsWe gratefully acknowledged the National Natural Science Foundation of China (Nos. 21772137, 21672157, 21372174), the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 16KJA150002), the Ph.D. Programs Foundation of PAPD, the project of scientific and technologic infrastructure of Suzhou (No. SZS201708), and Soochow University for financial support. We thank Jing-Hao Li in this group for reproducing the result of 3c, 3f, 4j and 3b'.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.08.006.
| [1] |
(a) M. Hutchby, Novel Synthetic Chemistry of Ureas and Amides, Springer, New York, 2013; (b) D.Q. Dong, S.H. Hao, H. Zhang, Z.L. Wang, Chin. Chem. Lett. 28 (2017) 1597-1599. |
| [2] |
(a) J. Zhang, S.H. Hong, Org. Lett. 14 (2012) 4646-4649; (b) W.D. Figg, S. Raje, K.S. Bauer, et al., J. Pharm. Sci. 88 (1999) 121-125; (c) S. Markonkawkeyoon, R.N.R. Limson, A.L. Moreira, V. Schauf, G. Kaplan, Proc. Natl. Acad. Sci. U. S. A. 90 (1993) 5974-5978; (d) R. Cumin, E.F. Bandle, E. Gamzu, W.E. Haefely, Psychopharmacology 78 (1982) 104-111; (e) J. Kang, V.K.R. Tangadanchu, L. Gopala, et al., Chin. Chem. Lett. 28 (2017) 1369-1374. |
| [3] |
(a) N.A. Al-Awadi, J. Chem. Soc. Perkin Trans. 2 (1990) 2187-2189; (b) P.Y. Reddy, S. Kondo, T. Toru, Y. Ueno, J. Org. Chem. 62 (1997) 2652-2654. |
| [4] |
(a) K.C. Nicolaou, C.J.N. Mathison, Angew. Chem. Int. Ed. 44 (2005) 5992-5997; (b) W.H. Huang, M.L. Wang, H. Yue, Synthesis 9 (2008) 1342-1344; (c) X. Ye, C.S. Xie, Y.Y. Pan, L.H. Han, T. Xie, Org. Lett. 12 (2010) 4240-4243; (d) V.K. Robert, S.G. Ning, R.S. Ronald, J.W. Lawrence, J. Am. Chem. Soc. 128 (2006) 5695-5702; (e) T. Kaicharla, M. Thangaraj, A.T. Biju, Org. Lett. 16 (2014) 1728-1731; (f) J. Kim, S.H. Hong, Org. Lett. 16 (2014) 4404-4407; (g) T.B. Nguyen, J. Sorres, M.Q. Tran, L. Ermolenko, A. Al-Mourabit, Org. Lett. 14 (2012) 3202-3205. |
| [5] |
(a) X.F. Wu, H. Neumann, M. Beller, Chem. Rev. 113 (2013) 1-35; (b) X.F. Wu, H. Neumann, ChemCatChem 4 (2012) 447-458; (c) Q. Liu, H. Zhang, A.W. Lei, Angew. Chem. Int. Ed. 50 (2011) 10788-10799; (d) X.F. Wu, H. Neumann, M. Beller, ChemSusChem 6 (2013) 229-241; (e) C.H. Schiesser, U. Wille, H. Matsubara, I. Ryu, Acc. Chem. Res. 40 (2007) 303-313; (f) S. Sumino, A. Fusano, T. Fukuyama, I. Ryu, Acc. Chem. Res. 47 (2014) 1563-1574; (g) I. Ryu, N. Sonoda, Angew. Chem. Int. Ed. 35 (1996) 1050-1066; (h) I. Ryu, Chem. Rec. 2 (2002) 249-258. |
| [6] |
(a) H.Q. Li, K.W. Dong, H. Neumann, M. Beller, Angew. Chem. Int. Ed. 54 (2015) 10239-10243; (b) Y.H. Li, K.W. Dong, F.X. Zhu, Z.C. Wang, X.F. Wu, Angew. Chem. Int. Ed. 55 (2016) 7227-7230; (c) L.J. Lu, D.Y. Cheng, Y.F. Zhan, et al., Chem. Commun. 53 (2017) 6852-6855. |
| [7] |
(a) J. Li, Y. He, S. Luo, et al., J. Org. Chem. 80 (2015) 2223-2230; (b) Y.Y. Pan, Y.N. Wu, Z.Z. Chen, et al., J. Org. Chem. 80 (2015) 5764-5770; (c) Q. Yang, C. Li, M.X. Cheng, S.D. Yang, ACS Catal. 6 (2016) 4715-4719; (d) Z.B. Chen, Y. Zhang, Q. Yuan, et al., J. Org. Chem. 81 (2016) 1610-1616; (e) Q. Zheng, Q. Ding, C. Wang, W. Chen, Y. Peng, Tetrahedron 72 (2016) 952-958; (f) J. Peng, Y. Gao, W. Hu, et al., Org. Lett. 18 (2016) 5924-5927; (g) G. Qiu, Q. Wang, J. Zhu, Org. Lett. 19 (2017) 270-273; (h) X. Huang, S. Xu, Q. Tan, et al., Chem. Commun. 50 (2014) 1465-1468; (i) S. Kim, S.H. Hong, Adv. Synth. Catal. 357 (2015) 1004-1012; (j) K. Takamatsu, K. Hirano, M. Miura, Org. Lett. 17 (2015) 4066-4069; (k) J. Lei, X. Wu, Q. Zhu, Org. Lett. 17 (2015) 2322-2325; (l) D. Li, T. Mao, J. Huang, Q. Zhu, Chem. Commun. 53 (2017) 1305-1308; (m) J. Liu, Z. Liu, P. Liao, et al., Angew. Chem. Int. Ed. 54 (2015) 10618-10622; (n) H. Wang, R.K. Kumar, Y. Yu, et al., Chem. Asian J. 11 (2016) 2841-2845; (o) Z. Hu, J. Dong, Y. Men, et al., Angew. Chem. Int. Ed. 56 (2017) 1805-1809; (p) K. Sugano, T. Tanase, K. Kobayashi, Y. Yamamoto, Chem. Lett. (1991) 921-924; (q) Q. Gao, P. Zhou, F. Liu, et al., Chem. Commun. 51 (2015) 9519-9522. |
| [8] |
(a) Z.Y. Gu, T.H. Zhu, J.J. Cao, et al., ACS Catal. 4 (2014) 49-52; (b) T.H. Zhu, S.Y. Wang, G.N. Wang, S.J. Ji, Chem. Eur. J. 19 (2013) 5850-5853; (c) T.H. Zhu, X.P. Xu, J.J. Cao, et al., Adv. Synth. Catal. 356 (2014) 509-518; (d) T.H. Zhu, S.Y. Wang, T.Q. Wei, S.J. Ji, Adv. Synth. Catal. 357 (2015) 823-828; (e) T.H. Zhu, S.Y. Wang, Y.Q. Tao, T.Q. Wei, S.J. Ji, Org. Lett.16 (2014) 1260-1263; (f) P. Xu, T.H. Zhu, T.Q. Wei, S.Y. Wang, S.J. Ji, RSC Adv. 6 (2016) 32467-32470; (g) P. Xu, F. Wang, T.Q. Wei, et al., Org. Lett. 19 (2017) 4484-4487; (h) Y. Fang, Z.L. Zhu, P. Xu, S.Y. Wang, S.J. Ji, Green Chem. 19 (2017) 1613-1618. |
| [9] |
(a) D.P. Curran, H. Liu, J. Am. Chem. Soc. 113 (1991) 2127-2132; (b) D.P. Curran, H. Liu, J. Am. Chem. Soc. 114 (1992) 5863-5864; (c) H. Josien, D. Bom, D.P. Curran, Y.H. Zheng, T.C. Chou, Bioorg. Med. Chem. Lett. 7 (1997) 3189-3194; (d) H. Josien, S.B. Ko, D. Bom, D.P. Curran, Chem. Eur. J. 4 (1998) 67-83; (e) B. Janza, A. Studer, Org. Lett. 8 (2006) 1875-1878; (f) T. Mitamura, K. Iwata, A. Ogawa, Org. Lett. 11 (2009) 3422-3424; (g) T. Mitamura, A. Ogawa, J. Org. Chem. 76 (2011) 1163-1166; (h) B. Zhang, A. Studer, Org. Lett. 16 (2014) 1216-1219. |
| [10] |
(a) J.J. Cao, T.H. Zhu, Z.Y. Gu, et al., Tetrahedron 70 (2014) 6985-6990; (b) J.J. Cao, X. Wang, S.Y. Wang, S.J. Ji, Chem. Commun. 50 (2014) 12892-12895. |
| [11] |
(a) A.S. Demir, O. Reis, M. Emrullahoglu, J. Org. Chem. 68 (2003) 578-580; (b) H. Wang, Y. Yu, X.H. Hong, B. Xu, Chem. Commun. 50 (2014) 13485-13488; (c) X.M. Huang, S.G. Xu, Q.T. Tan, et al., Chem. Commun. 50 (2014) 1465-1468; (d) G.B. Yan, M.H. Yang, X.M. Wu, Org. Biomol. Chem. 11 (2013) 7999-8008. |
| [12] |
(a) Y. Wang, M. Li, J. Peng, C. Chen, Angew. Chem. Int. Ed. 52 (2013) 5323-5327; (b) X. Su, M. Li, Y. Wang, et al., Chem. Commun. 49 (2013) 6752-6754; (c) C.K. Cao, J.Y. Sheng, C. Chen, Synthesis 49 (2017) 5081-5092; (d) Y. Wang, X. Su, C. Chen, Synlett 24 (2013) 2619-2623. |
 2019, Vol. 30
2019, Vol. 30 


