The control of reaction conditions for selective synthesis of diverse target products from the same reactants has attracted continuous interest in synthetic chemistry [1]. Various exciting studies using this strategy have recently been reported and researchers have shown great enthusiasm for the synthesis of many different compounds [2]. Biju and coworkers [3] demonstrated the temperature-dependent highly selective synthesis of N-unsubstituted and N-arylindoles using the same starting materials. In addition, Sun and Ji [4] developed the tunable synthesis of alkenes or ketones from cinnamic acids and alkanes in the presence of di-tert-butyl peroxide (DTBP) or tert-butyl hydroperoxide (TBHP). The different resultant products were obtained simply by varying the peroxide. Furthermore, Yuan and his colleagues [5] also reported a new method for the reaction of β- arylsulfonyl enamines and sulfonamides that depends on the reaction solvent. The reactions proceeded smoothly via I2/TBHPmediated C-H and C-N bond cleavage of tertiary amines. It is noteworthy that the selective synthesis of different target products can be achieved under the same conditions or with the same substrates through a subtle change [6] in time, temperature, solvent, or oxidant. The exploration of new highly selective metalfree methods to access various products under the same conditions is still highly valuable but full of challenges.
α-Ketoamides and quinoxalines exist in a broad range of bioactive natural compounds [7] and play an important role in the intermediates of organic synthesis. α-Ketoamide compounds are known to possess widespread and powerful pharmacologic properties. Quinoxaline derivatives have antimicrobial, anticancer, antibacterial, and anti-inflammatory properties [8] due to their chemical and biological activities. Many methods have been described for the synthesis of α-ketoamides and quinoxalines. In addition, many α-ketoamide production routes have been found and are based on a broad range of starting materials, such as styrene or phenylacetylene [9], hypnone [10] and phenyl oxalaldehyde [11], and the construction of the quinoxalines are based on the above starting materials too (Scheme 1). In this report, we disclose a mild and highly efficient method, to the best of our knowledge, which serves as the first reliable, base-free method to selectively prepare either α-ketoamides or quinoxalines by simple variation of the substitution number of amino groups at room temperature under visible light irradiation. This protocol has the advantages of readily available starting materials, no additives and high yield.
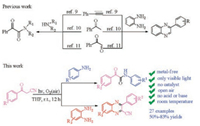
|
Download:
|
| Scheme 1. Synthesis of α-ketoamides and quinoxalines. | |
We first commenced our study with benzoylacetonitrile and aniline, exposing to a halogen tungsten lamp (500 W) at room temperature. The desired product α-ketoamides was formed in 52% yield after 8 h in THF (Table 1, entry 1). Then control experiment was performed in the absence of visible light (Table 1, entry 2). The results showed that the visible light is essential for the formation of the desired product. Next we study the effect of other parameters on the reaction, including reaction time, molar ratio and solvent. By decreasing the reaction time to 4 h, the product yield was decreased to 21% while increasing the reaction time to 12 h, which was increased to 59% (Table 1, entries 3 and 4). However, an additional increase in the reaction time did not produce the higher yield (Table 1, entry 5). Then the diverse molar ratio of benzoyl acetonitrile and aniline was studied under the same conditions except reaction time. First, we studied the reaction at different reaction time under the molar ratio is 1:1.5, interestingly, the product yield increased from 61% to 72% and did not change with the time extending to 20 h, which indicate that 12 h may be enough for the substrate to react with each other (Table 1, entries 6–8). No significant improvement of yield was observed when increase the molar ratio above 1:1.5 (Table 1, entries 9 and 10). To further enhance the α-ketoamide yield, an extensive screening of solvents was tested. Among various tested solvents, including DMSO, DMF, DCM, NMP, MeCN, toluene, the yield of 3a was lower than in THF (Table 1, entry 7 and entries 11–16), which indicates that the reaction is sensitive to reaction media. THF was the best solvent in terms of the reaction yield, hence it was used throughout the present work.
|
|
Table 1 Optimization of reaction conditions.a |
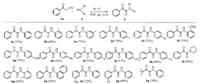
To confirm whether diverse α-ketoamides could be synthesized through the optimized method, the scope of the substrates was investigated. As summarized in Fig. 1, the reaction showed good compatibility and proved to be a general method to construct diverse α-ketoamides. Aniline containing both electron-donating and electron-withdrawing groups reacted with benzoylacetonitrile to afford the desired products in moderate to good yields (3a-3n). The substituents at different positions did not greatly affect the yield (3b-3e, 68%–78%). Aniline bearing electron-neutral (H, 4-Me, Et) and electron-donating (OMe and t-Bu) groupsresulted in successful conversion to the corresponding products in moderate to good yields (3a and 3f–3h, 64%–79%). The scope of the substrates was further extended to various electron-withdrawing halogenated substrate (F, CF3, Cl), giving the corresponding products with 54%–60% yields (3i-3k) successfully. A satisfactory yield was also achieved using secondary amines as the products (3l, 83%). Additionally, 1-naphthylamine also proceeded smoothly to generate the corresponding product 3n with a 60% yield under the standard reaction conditions. Moreover, benzoylacetonitrile bearing electron-neutral and electron-withdrawing groups or aliphatic acyl acetonitrile reacted well with aniline (3o-3q, 68%–78%).
|
Download:
|
| Fig. 1. Substrate scope for α-ketoamides synthesis. Reaction conditions: 1a (0.5 mmol), 2 (0.75 mmol), solvent (2 mL), room temperature. Isolated yields in parentheses. | |
When using 1, 2-diaminobenzene to replace aniline, the experimental products were quite different from our expected results (Scheme S1 in Supporting information). We synthesized quinoxalines from 1, 2-diaminobenzene and benzoylacetonitrile under our optimized reaction conditions with 76% yield and without by-product. We then expanded the scope of benzoylacetonitrile. As shown in Fig. 2, the reactions were also highly efficient and smooth with yields of 63% to 72% (5b-5d), both electronwithdrawing and electron-donating groups have a relatively low impact on the reaction rate and yield. 2-Thenoylacetonitrile can also give the corresponding product with 50% yield (5f). We also scope the 1, 2-diaminobenzene with electron-withdrawing and electron-donating groups, to our satisfactory, the substituted 1, 2- diaminobenzene also proceed smoothly with benzoylacetonitrile. It is noteworthy that under the circumstances of single substituted 1, 2-diaminobenzene, two regioisomers were obtained due to the unsymmetric nature of single substituted 1, 2-diaminobenzene (5g-5i), which react equally well to the C–O double bond. While the symmetric substituted 1, 2-diaminobenzene only give one product (5j). Importantly, the present method for generating quinoxalines proceeds without the use of a metal or an extra oxidant.

|
Download:
|
| Fig. 2. Substrate scope for α-ketoamides synthesis. Reaction conditions: 1 (0.5 mmol), 4a (0.75 mmol), solvent (2 mL), room temperature. Isolated yields in parentheses. | |
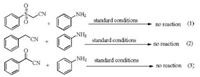
In order to further explore the active site of the reaction, we conducted the following control experiments (Scheme 2). When we converted the carbonyl to sulfone, no reaction was detected, so carbonyl is important in the reaction. At the same time, neither benzoylcyanide nor benzonitrile was found to react with aniline under this condition, indicating that the cyanogroup and methylene were equally important.
|
Download:
|
| Scheme 2. Control experiments. | |
Although we knew that visible light and O2 were necessary for the success of the reaction, we wondered what is the key factor enabled this catalyst-free process. One possibility is that singlet oxygen is produced and responsible for the oxidative coupling process under our standard conditions. Experiments to determine the presence of photoexcited singlet oxygen in the reaction system were performed (Scheme S2 in Supporting information). Trapping experiments were carried under irradiation using 1, 3-diphenylisobenzofuran [12] produced the desired product 1, 2-phenylenebis (phenylmethanone), however, the corresponding product was not acquired in the dark, which suggested that under our reaction conditions, visible-light promoted the generation of singlet oxygen. Quenching experiments were conducted, using 1, 4- diazabicyclo[2.2.2]octane (DABCO) [13], a common quencher of 1O2, only a trace amount of 3a was produced in the reaction. These quenching and trapping experiments clearly indicated that 1O2 was generated from O2 and acted as an indispensable reactant in our system.
Based on the above results and the recent research by Xu [14], a possible reaction mechanism for α-ketoamide and quinoxalines was obtained and illustrated below (Scheme 3). We reasoned that benzoylacetonitrile and oxygen react via visible light. Singlet oxygen that is generated from oxygen by visible-light stimulation then extracts an electron from benzoylacetonitrile A to generate B, which reacted with HOO radical to give intermediate C, then intermediate C underwent dehydration to form the intermediate D, which then reacts with amines to produce either α-ketoamides or quinoxalines through two pathways: react with aniline to give intermediate E and cyanohydrination of E to lead to the final product 3a [15]. While react with 1, 2-diaminobenzene underwent condensation to produce the product 5a.

|
Download:
|
| Scheme 3. A plausible mechanism. | |
In conclusion, we have descripted a novel and efficient visiblelight-promoted protocol for selectively preparing α-ketoamides and quinoxalines under mild conditions. This work is the first to demonstrate that the selectivity of target products between primary amines and benzoylacetonitrile can be controlled by changing the position of the amino group. The reaction takes place smoothly to generate the corresponding products in moderate to good yields. It is a novel protocol that accommodates different anilines and benzoylacetonitrile through visible-light induced electron transfer and oxidative coupling. The reactivity, in the absence of photosensitizer, further improves its practicality. Studies of the detailed mechanism of this process and its application are underway.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j. cclet.2018.06.019.
| [1] |
(a) S. Arseniyadis, A. Valleix, A. Wagner, C. Mioskowski, Angew. Chem. Int. Ed. 43 (2004) 3376-3379; (b) B.M. Trost, A. Fettes, B.T. Shireman, J. Am. Chem. Soc. 126 (2004) 2660-2661; (c) G. Zanoni, F. Castronovo, M. Franzini, G. Vidari, E. Giannini, Chem. Soc. Rev. 32 (2003) 115-129. |
| [2] |
(a) C.C. Malakar, D. Schmidt, J. Conrad, U. Beifuss, Org. Lett. 13 (2011) 1972-1975; (b) J.B. Gibbons, J.M. Salvant, R.M. Vaden, et al., J. Org. Chem. 80 (2015) 10076-10085; (c) Y.L. Zhu, B. Jiang, W.J. Hao, et al., Org. Lett. 17 (2015) 6078-6081. |
| [3] |
M. Thangaraj, S.S. Bhojgude, S. Jain, R.G. Gonnade, A.T. Biju, J. Org. Chem. 81 (2016) 8604-8611. DOI:10.1021/acs.joc.6b01472 |
| [4] |
J. Ji, P. Liu, P. Sun, Chem. Commun. 51 (2015) 7546-7549. DOI:10.1039/C5CC01762A |
| [5] |
J. Lai, L. Chang, G. Yuan, Org. Lett. 18 (2016) 3194-3197. DOI:10.1021/acs.orglett.6b01412 |
| [6] |
(a) J.D. Hu, C.P. Cao, W. Lin, et al., J. Org. Chem. 79 (2014) 7935-7944; (b) J.H. Mao, Z.T. Wang, Z.Y. Wang, Y. Cheng, J. Org. Chem. 80 (2015) 6350-6359; (c) S.F. Pi, X.H. Yang, X.C. Huang, et al., J. Org. Chem. 75 (2010) 3484-3487. |
| [7] |
(a) Y.H. Chen, Y.H. Zhang, H.J. Zhang, et al., J. Med. Chem. 49 (2006) 1613-1623; (b) Y. Hu, J.B. MacMillan, Org. Lett. 13 (2011) 6580-6583; (c) T.D. Ocaint, D.H. Rich, J. Med. Chem. 35 (1992) 451-456; (d) W.P. Mai, H.H. Wang, Z.C. Li, et al., Chem. Commun. 48 (2012) 10117-10119. |
| [8] |
(a) L.E. Seitz, W.J. Suling, R.C. Reynolds, J. Med. Chem. 45 (2002) 5604-5606; (b) J. Zhang, Y. Wei, S. Lin, F. Liang, P. Liu, Org. Biomol. Chem. 10 (2012) 9237-9242; (c) W. He, M.R. Myers, B. Hanney, et al., Bioorg. Med. Chem. Lett. 13 (2003) 3097-3100; (d) Y.B. Kim, Y.H. Kim, J.Y. Park, S.K. Kim, Bioorg. Med. Chem. Lett. 14 (2004) 541-544. |
| [9] |
(a) R. Deshidi, S. Devari, B.A. Shah, Eur. J. Org. Chem. 2015 (2015) 1428-1432; (b) R. Deshidi, M. Kumar, S. Devari, B.A. Shah, Chem. Commun. 50 (2014) 9533-9535; (c) K.S. Vadagaonkar, H.P. Kalmode, K. Murugan, A.C. Chaskar, RSC Adv. 5 (2015) 5580-5590. |
| [10] |
(a) K. Padmavathy, G. Nagendrappa, K.V. Geetha, Tetrahedron. Lett. 52 (2011) 544-547; (b) Z. Zhang, J. Su, Z. Zha, Z. Wang, Chem. Commun. 49 (2013) 8982-8984. |
| [11] |
(a) K. Aghapoor, F. Mohsenzadeh, A. Shakeri, et al., J. Organomet. Chem. 743 (2013) 170-178; (b) A.K. Padala, N. Mupparapu, D. Singh, R.A. Vishwakarma, Q.N. Ahmed, Eur. J. Org. Chem. 2015 (2015) 3577-3586. |
| [12] |
H. Wang, X. Yang, W. Shao, et al., J. Am. Chem. Soc. 137 (2015) 11376-11382. DOI:10.1021/jacs.5b06025 |
| [13] |
M. Klaper, W. Fudickar, T. Linker, J. Am. Chem. Soc. 138 (2016) 7024-7029. DOI:10.1021/jacs.6b01555 |
| [14] |
W.T. Xu, B. Huang, J.J. Dai, J. Xu, H.J. Xu, Org. Lett. 18 (2016) 3114-3117. DOI:10.1021/acs.orglett.6b01296 |
| [15] |
Z. Deng, X. Lu, Z. Wen, et al., RSC. Adv. 4 (2014) 12266-12274. DOI:10.1039/c3ra46544f |
 2019, Vol. 30
2019, Vol. 30 


