Spirocyclic structures exist in many natural products [1] and numerous spirocyclic compounds display various useful pharmacological and therapeutical activities [2]. Generally, spiro structures are constructed by forming a new ring on an existing carboor heterocycle [3]. These strategies include alkylation methods [4], transition-metal based processes [5], radical cyclizations [6], ring closure of geminally substituted compounds [7], metathesis processes [8], Diels-Alder reactions [9], cycloaddition reactions [10], rearrangement based processes [11], etc. In some limited examples, two rings of spirocyclic compounds were assembled in one-pot through a double intramolecular 1, 3-dipolar cycloaddition of diene [12]. In 1994, Zecchi reported double [3 + 2] cycloaddition between sulfonylallenes and nitrile oxides for synthesis of spirocyclic compounds, but the yields were very low and the chemoselectivity was extremely poor [13]. Most recently, we developed a facile strategy that both rings of spirocyclic compounds were established in one-pot via the double 1, 3-dipolar cycloaddition of both carbon-carbon double bonds of allenoates (Scheme 1a). The spirobidihydropyrazoles were synthesized in moderate to excellent yields with excellent diastereoselectivitie through the double [3 + 2] cycloaddition of allenoates with nitrilimines under mild reaction conditions [14a]. In order to further use this strategy to synthesize biologically aimportant siprocyclic compounds, herein, we report synthesis of the spirobidihydroisoxazoles [15] through the double [3 + 2] cycloaddition of allenoates with nitrile oxides (Scheme 1b).
|
Download:
|
| Scheme 1. Synthesis of spirocyclic heterocyclic compounds via double [3 + 2] cycloaddition of allenoates. | |
As a type of readily accessible 1,3-dipoles, nitrile oxides have extensively been utilized in various cycloaddition reactions [16]. Generally, nitrile oxides are unstable and thus are employed as reactive species produced in situ from the corresponding oxime halides in the presence of a base. Exceptionally, some nitrile oxides are stable and can be isolated. In our investigation, the stable nitrile oxide 1a was used (Table 1). Initially, the double [3 + 2] cycloaddition of nitrile oxide 1a with allenoate 2a was examined in dichloromethane at room temperature, and delightedly, the desired spirobidihydroisoxazole 3aa was obtained in 59% yield after 84 h of reaction (Table 1, entry 1). In order to increase the yield, we screened several solvents (entries 2–8). Except for methanol, other solvents were compatible with the reaction, leading to the product 3aa in acceptable yields (entries 2–8). Particularly, the highest 78% yield of the product 3aa was obtained in the case of tetrahydrofuran (THF) as solvent (entry 2). With the use of THF as the solvent, we next explored the influence of the reaction temperature (entries 9–11). The experimental results showed that although increasing temperature could remarkably accelerate the reaction and thus shorten the reaction time, the yield remained the nearly same as that at room temperature. TLC monitoring displayed that the product decomposed to some side product at high temperature. When the input of the nitrile oxide 1a was increased, the reaction time was further reduced to 24 h (entry 12). The structure and relative configuration of the cycloadduct has been determined through X-ray crystallographic data of the product 3aa. And the crystallographic data have been deposited with the Cambridge Crystallographic Data Centre with CCDC No. 1588797.
|
|
Table 1 Screening of the reaction conditions.a |
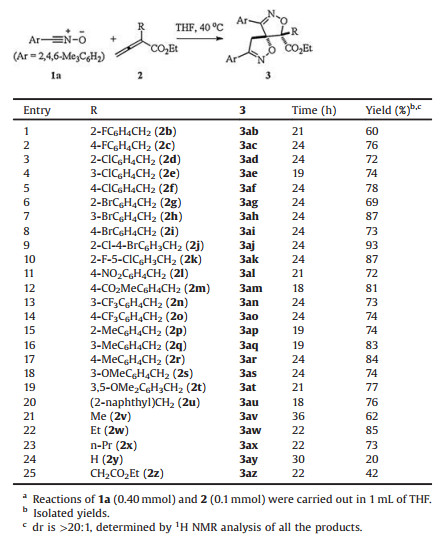
Under the optimized reaction conditions established, various allenoates 2 were explored in the double [3 + 2] cycloaddition with nitrile oxide (Table 2). The allenoates bearing whether the electron-withdrawing or electron-donating group on the benzene ring, worked well in the reaction at 40℃ to produce the products 3 in moderate to excellent yields (60%–93%) with excellent diastereoselectivities (entries 1–19). In general, the position of the substituent on the benzene ring has a certain degree of impact on the yield. The allenoate having substituent at 2-position of benzene led to lower yield than their corresponding isomer bearing substituent at 3 or 4-position of benzene did (entry 1 vs. 2, 3 vs. 4 or 5, 6 vs. 7 or 8, 15 vs. 16 or 17). Notably, the bihalogen-substituted allenoates resulted in good to excellent yields (entries 9 and 10). 2-Naphthyl-substituted allenoate also performed the reaction to give the desired product in 76% yield (entry 20). Meanwhile, α-alkyl-substituted allenoates were compatible substrates as well, affording the corresponding product 3 in 62%–85% yields (entries 21–23). In addition, unsubstituted allenoate (2y) and β-ethoxycarbonyl-substituted allenoate (2z) were also examined, giving the corresponding product 3 in 20% yield and 42% yield, respectively (entries 24 and 25).
|
|
Table 2 Scope of allenoate.a |
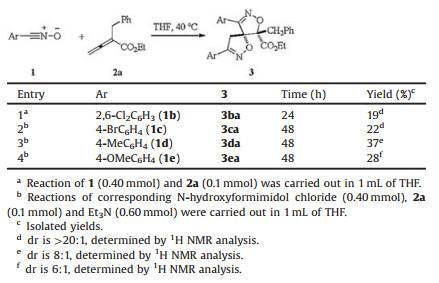
Next, we carried out an investigation on the scope of nitrile oxide 1 (Table 3). The stable nitrile oxide 1b performed the double [3 + 2] cycloaddition with allenoates, leading to the corresponding product 3ba in 19% yield with excellent diastereoselectivity (entry 1). The unstable nitrile oxides 1c, 1d and 1e generated in situ from the corresponding oxime halides in the presence of a base, also worked in this reaction, giving the desired products 3ca, 3da and 3ea in 22%, 37% and 28% yield, respectively (entries 2–4).
|
|
Table 3 Scope of nitrile oxide. |
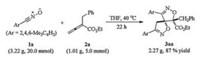
As indicated in Scheme 2, a gram-scale preparation of the product 3aa was also performed. The nitrile oxide 1a (20.0 mmol) reacted with allenoate 2a (1.01 g, 5.0 mmol) under the standard reaction conditions to produce the spirobidi-hydroisoxazole 3aa in 87% yield with excellent regioselectivity, demonstrating the practicability of the current reaction.
|
Download:
|
| Scheme 2. Synthesis on the gram scale. | |
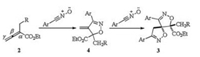
As shown in the Scheme 3, the reaction of allenoates with nitrile oxides undergoes a sequential double [3 + 2] cycloaddition. This is supported by the intermediate dihydroisoxazole 4, which has been isolated and characterized. The mechanism is the same as that in our previous work [14a]. The [3 + 2] cycloaddition of nitrile oxide with the carbon-carbon double bond of allenoate between the α and β-carbon, followed by another [3 + 2] cycloaddition of second nitrile oxide with the terminal olefin in the intermediate 4, leads to the spirocyclic heterocyclic compounds 3 bearing two chiral quaternary carbon centers.
|
Download:
|
| Scheme 3. The mechanism involving sequential double [3 + 2] cycloaddition. | |
In conclusion, the double [3 + 2] cycloaddition of allenoates with nitrile oxides has been accomplished under mild reaction conditions for synthesis of spirobidihydroisoxazole. Various a-substituted allenoates conducted the reaction to provide the desired spirobidihydroisoxazole in moderate to excellent yields with excellent diastereoselectivities. Further studies regarding the bioactivity of the spirobidihydroisoxazoles are currently underway in our laboratory.
AcknowledgmentThis work is supported by the National Natural Science Foundation of China (Nos. 21372256, 21572264)
| [1] |
(a) M. Sannigrahi, Tetrahedron 55 (1999) 9007-9071; (b) S. Kotha, A.C. Deb, K. Lahiri, E. Manivannan, Synthesis 2 (2009) 165-193; (c) O.O. Grygorenko, D.S. Radchenko, D.M. Volochnyuk, A.A. Tolmachev, I.V. Komarov, Chem. Rev. 111 (2011) 5506-5568. |
| [2] |
(a) P.R. Berquist, R.J. Wells, Bioactive marine biopolymers, in: P.J. Scheuer (Ed.), Marine Natural Products, Elsevier Inc., New York, 1983, pp. 391-427; (b) F. Perron, K.F. Albizati, Chem. Rev. 89 (1989) 1617-1661; (c) P.R. Berquist, R.J. Wells, Chemotaxonomy of the porifera: the development and current status of the field, in: P.J. Scheuer (Ed.), Marine Natural Products, Elsevier Inc., New York, 1983, pp. 1-50; (d) G.M. Konig, A.D. Wright, Heterocycles 36 (1993) 1351-1358; (e) A.A. Alahmadi, M.F. El-zohryt, J. Chem. Technol. Biotechnol. 62 (1995) 366-372; (f) G.L. Arutyunyan, A.A. Chachoyan, T.E. Agadzhanyan, B.T. Garibdzhanyan, Pharm. Chem. J. 30 (1996) 739-741; (g) M.H. Chen, P.P. Pollard, A.A. Patchett, et al., Bioorg. Med. Chem. Lett. 9 (1999) 1261-1266; (h) R.D. Encarnación, E. Sandoval, J. Malmstrøm, C. Christophersen, J. Nat. Prod. 63 (2000) 874-875; (i) V.M. Kisel, E.O. Kostyrko, V.A. Kovtunenko, Chem. Heterocycl. Comp. 38 (2004) 1295-1318; (j) B.S. Lukyanov, M.B. Lukyanov, Chem. Heterocycl. Comp. 41 (2006) 281-311; (k) Ö. Güzel, E. Ilhan, A. Salman, Monatsh. Chem. 137 (2006) 795_-801; (l) N.S. Joshi, B.K. Karale, C.H. Gill, Chem. Heterocycl. Compd. 42 (2006) 681-685; (m) H. Habib-Zahmani, J. Viala, S. Hacini, J. Rodriguez, Synlett 7 (2007) 1037-1042. |
| [3] |
(a) S. Kotha, A.C. Deb, K. Lahiri, E. Manivannan, Synthesis 2 (2009) 165-194; (b) R. Rios, Chem. Soc. Rev. 41 (2012) 1060-1074; (c) Z.Y. Cao, X. Wang, C. Tan, et al., J. Am. Chem. Soc. 135 (2013) 8197-8200; (d) Y.L. Liu, X. Wang, Y.L. Zhao, et al., Angew. Chem. Int. Ed. 52 (2013) 13735-13739; (e) X.P. Yin, X.P. Zeng, Y.L. Liu, et al., Angew. Chem. Int. Ed. 53 (2014) 13740-13745; (f) J.S. Yu, F.M. Liao, W.M. Gao, et al., Angew. Chem. Int. Ed. 54 (2015) 7381-7385. |
| [4] |
A.P. Krapcho, Synthesis 6 (1974) 383-419. |
| [5] |
(a) J.A. Palmes, A. Aponick, Synthesis 44 (2012) 3699-3721; (b) V.A. D'Yakonov, O.A. Trapeznikova, A. de Meijere, U.M. Dzhemilev, Chem. Rev. 114 (2014) 5775-5814; (c) Z.Y. Cao, J. Zhou, Org. Chem. Front. 2 (2015) 849-858; (d) T. Nemoto, Y. Hamada, Synlett 27 (2016) 2301-2313. |
| [6] |
J. Sperry, Y.C. Liu, M.A. Brimble, Org. Biomol. Chem. 8 (2010) 29-38. DOI:10.1039/B916041H |
| [7] |
L.K. Smith, I.R. Baxendale, Org. Biomol. Chem. 13 (2015) 9907-9933. DOI:10.1039/C5OB01524C |
| [8] |
(a) K. Undheim, J. Efskind, Tetrahedron 56 (2000) 4847-4857; (b) N.Y. Kuznetsov, Y.N. Bubnov, Russ. Chem. Rev. 84 (2015) 758-785. |
| [9] |
M.A. Rizzacasa, A. Pollex, Org. Biomol. Chem. 7 (2009) 1053-1059. DOI:10.1039/b819966n |
| [10] |
(a) G.P. Savage, Curr. Org. Chem. 14 (2010) 1478-1499; (b) N. Arumugam, R.S. Kumar, A.I. Almansour, S. Perumal, Curr. Org. Chem. 17 (2013) 1929-1956; (c) E.M. Hussein, 1, 3-Dipolar cycloadditions approach to bioactive spiroheterocycles, in: K.L. Ameta, R.P. Pawar, A.J. Domb (Eds.), Bioactive Heterocycles: Synthesis and Biological evaluation, Nova Science Publishers Inc., New York, 2013, pp. 157-186; (d) P.W. Xu, J.K. Liu, L. Shen, et al., Nat. Commun. 8 (2017) 1619-1626; (e) Y.N. Gao, M. Shi, Chin. Chem. Lett. 28 (2017) 493-502. |
| [11] |
(a) A.P. Krapcho, Synthesis 7 (1976) 425-444; (b) A. Nakazaki, S. Kobayashi, Synlett 23 (2012) 1427-1445. |
| [12] |
(a) M.A. Arai, T. Arai, H. Sasai, Org. Lett. 1 (1999) 1795-1797; (b) M.A. Arai, M. Kuraishi, T. Arai, H. Sasai, J. Am. Chem. Soc. 123 (2001) 2907-2908. |
| [13] |
G. Broggini, G. Molteni, G. Zecchi, J. Org. Chem. 59 (1994) 8271-8274. DOI:10.1021/jo00105a055 |
| [14] |
(a) H.L. Liu, H. Jia, B. Wang, Y.M. Xiao, H.C. Guo, Org. Lett. 19 (2017) 4714-4717; (b) R.S. Na, C.F. Jing, Q.H. Xu, et al., J. Am. Chem. Soc. 133 (2011) 13337-13348; (c) R.S. Na, H.L. Liu, Z. Li, et al., Tetrahedron 68 (2012) 2349-2356; (d) J. Liu, H.L. Liu, R.S. Na, et al., Chem. Lett. 41 (2012) 218-220; (e) C.F. Jing, R.S. Na, B. Wang, et al., Adv. Synth. Catal. 354 (2012) 1023-1034; (f) X. Wu, R.S. Na, H.L. Liu, et al., Tetrahedron. Lett. 53 (2012) 342-344; (g) L. Zhang, C.F. Jing, H.L. Liu, et al., Synthesis 45 (2013) 53-64; (h) Z. Li, H. Yu, L. Zhang, et al., Lett. Org. Chem. 11 (2014) 220-224; (i) L. Zhang, H.L. Liu, G.Y. Qiao, et al., J. Am. Chem. Soc. 137 (2015) 4316-4319; (j) Z. Li, H. Yu, Y.L. Feng, et al., RSC Adv. 5 (2015) 34481-34485; (k) F.L. Li, J.F. Chen, Y.D. Hou, et al., Org. Lett. 17 (2015) 5376-5379; (l) H.L. Liu, Y. Liu, C.F. Yuan, et al., Org. Lett. 18 (2016) 1302-1305; (m) C.H. Yuan, L.J. Zhou, M.R. Xia, et al., Org. Lett. 18 (2016) 5644-5647; (n) Z. Li, H. Yu, Y. Liu, et al., Adv. Synth. Catal. 358 (2016) 1880-1885; (o) X. Zhang, C.H. Yuan, C. Zhang, et al., Tetrahedron 72 (2016) 8274-8281; (p) L.J. Zhou, C.H. Yuan, C. Zhang, et al., Adv. Synth. Catal. 359 (2017) 2316-2321; (q) C. Wang, H. Jia, C. Zhang, et al., J. Org. Chem. 82 (2017) 633-641; (r) B. Mao, W. Shi, J. Liao, et al., Org. Lett. 19 (2017) 6340-6343; (s) B. Wang, H. Liu, Q. Wang, et al., Tetrahedron 73 (2017) 5926-5931; (t) W. Yang, W. Sun, C. Zhang, et al., ACS Catal. 7 (2017) 3142-3146; (u) L. Zhou, C. Yuan, Y. Zeng, et al., Chem. Sci. 9 (2018) 1831-1835. |
| [15] |
(a) A. Sysak, B. Obminska-Mrukowicz, Eur. J. Med. Chem. 137 (2017) 292-309; (b) K. Kaur, V. Kumar, A.K. Sharma, G.K. Gupta, Eur. J. Med. Chem. 77 (2014) 121-133; (c) G.N. Pairas, F. Perperopoulou, P.G. Tsoungas, G. Varvounis, ChemMedChem 12 (2017) 408-419; (d) A. Pinto, L. Tamborini, G. Cullia, P. Conti, C. De Micheli, ChemMedChem 11 (2016) 10-14; (e) S. Muthusamy, S.M. Lee, M. Huang, et al., Bull. Korean Chem. Soc. 37 (2016) 1020-1028; (f) J.E. Casida, Chem. Res. Toxicol. 28 (2015) 560-566; (g) F. Beugnet, J. Liebenberg, L. Halos, Vet. Parasitol. 209 (2015) 142-145; (h) C. Zhao, J.E. Casida, J. Agric. Food. Chem. 62 (2014) 1019-1024; (i) A. Pinto, P. Conti, M. De Amici, et al., J. Med. Chem. 51 (2008) 2311-2315. |
| [16] |
(a) H. Suga, Y. Hashimoto, Y. Toda, et al., Angew. Chem. Int. Ed. 56 (2017) 11936-11939; (b) V.A. Lofstrand, F.G. West, Chem. Eur. J. 22 (2016) 10763-10767; (c) K.S. Vinay Kumar, G.S. Lingaraju, Y.K. Bommegowda, et al., RSC Adv. 5 (2015) 90408-90421; (d) T. Ikawa, H. Kaneko, S. Masuda, et al., Org. Biomol. Chem. 13 (2015) 520-526; (e) Y. Yamashita, Y. Hirano, A. Takada, H. Takikawa, K. Suzuki, Angew. Chem. Int. Ed. 52 (2013) 6658-6661; (f) E.M. Beccalli, G. Broggini, M. Martinelli, N. Masciocchi, S. Sottocornola, Org. Lett. 8 (2006) 4521-4524; (g) O. Altintas, M. Glassner, C. Rodriguez-Emmenegger, et al., Angew. Chem. Int. Ed. 54 (2015) 5777-5783; (h) F. Friscourt, C.J. Fahrni, G.J. Boons, Chem. Eur. J. 21 (2015) 13996-14001; (i) B.K. Kuruba, S. Vasanthkumar, Tetrahedron 73 (2017) 3860-3865; (j) J.A. Crossley, D.L. Browne, J. Org. Chem. 75 (2010) 5414-5416; (k) H. Zheng, R. McDonald, D.G. Hall, Chem. Eur. J. 16 (2010) 5454-5460; (l) O. Jackowski, T. Lecourt, L. Micouin, Org. Lett. 13 (2011) 5664-5667; (m) X. Xu, D. Shabashov, P.Y. Zavalij, M.P. Doyle, Org. Lett. 14 (2012) 800-803; (n) G. Molteni, P. Del Buttero, Tetrahedron 67 (2011) 7343-7347; (o) M.A. Hofmann, U. Bergsträßer, G.J. Reiß, L. Nyulászi, M. Regitz, Angew. Chem. Int. Ed. 39 (2000) 1261-1263; (p) H. Suga, Y. Adachi, K. Fujimoto, et al., J. Org. Chem. 74 (2009) 1099-1113; (q) P. Quadrelli, M. Mella, S. Carosso, B. Bovio, P. Caramella, Eur. J. Org. Chem. 2007 (2007) 6003-6015; (r) C.K. Lee, A.B. Holmes, B. Al-Duri, et al., Chem. Commun. 2004 (2004) 2622-2623; (s) T. Rispens, J.B.F.N. Engberts, J. Org. Chem. 68 (2003) 8520-8528; (t) P. Quadrelli, V. Fassardi, A. Cardarelli, P. Caramella, Eur. J. Org. Chem. 2002 (2002) 2058-2065; (u) C. Zorn, B. Anichini, A. Goti, et al., J. Org. Chem. 64 (1999) 7846-7855; (v) A. Mack, B. Breit, T. Wettling, et al., Angew. Chem. Int. Ed. 36 (1997) 1337-1340; (w) R. Huisgen, R. Temme, Eur. J. Org. Chem. 1998 (1998) 387-401; (x) W.S. Chung, T.L. Tsai, C.C. Ho, M.Y.N. Chiang, W.J. le Noble, J. Org. Chem. 62 (1997) 4672-4676; (y) L. Bruche, M.L. Gelmi, G. Zecchi, J. Org. Chem. 50 (1985) 3206-3208. |
 2019, Vol. 30
2019, Vol. 30