b State Key Laboratory of Food Science & Technology, Wuxi 214122, China
At present, there has been growing interest in the development and utilization of green energy [1, 2]. Biomass, as the only renewable carbon resource, has been treated as an alternative resource for the production of liquid fuels and chemicals. In recent years, many value-added biomass platform molecules have been successfully synthesized from lignocellulosic biomass through different reactions, such as 5-hydroxymethylfurfural (HMF), lactic acid, levulinic acid and furfuryl alcohol [3-6]. Among them, HMF is a versatile and key intermediate in biofuel chemistry and petroleum industry [7, 8]. What is more, HMF can be further converted into more than 175 valuable biomass-derived products that can be used as fine chemicals, polymeric materials, pharmaceuticals and liquid fuels [9-12]. Therefore, the efficient conversion of carbohydrates to HMF is very important for the biomass utilization.
So far, one of the major routes for the production of HMF is based on acid catalytic dehydration of fructose [10]. In recent decades, a series of acid catalysts, such as various mineral acids, metal Lewis acids and acidic ionic liquids, have been successfully applied to the dehydration of fructose [13-16]. Apart from fructose, other sugars (e.g., glucose, sucrose, inulin, mannose, galactose and cellulose) could also be used to produce HMF. Zhang and Zhou reviewed one-pot catalytic conversion of carbohydrates into HMF, which described the conversion of inulin and glucose into HMF in detail [17]. In addition, Heeres et al. made a comprehensive review of HMF, including the preparation, application and current problems of HMF [18]. In order to improve the yield and selectivity of HMF, an efficient strategy for the direct production of HMF from fructose was proposed using ionic liquids CnMI·Cl (n = 4, 8, 10, 12 and 16) and HCl as catalyst system [19]. Jadhav et al. synthesized a series of asymmetrical dicationic ionic liquids and applied them to fructose dehydration [20]. Although ionic liquid has an excellent catalytic effect on the dehydration of fructose into HMF, it is difficult to separate and recover from the reaction liquid. Hence, seeking an efficient and recyclable catalyst for the conversion of fructose into HMF is necessary. Han et al. used reduced graphene oxide-supported tungsten trioxide (WO3/RGO) as an acidic catalyst and 84.2% yield of HMF was obtained from fructose [21]. Liu et al. found that the conversion of fructose into HMF was catalyzed by K-10 clay-Al in DMSO with 93.2% HMF yield, where DMSO also inhibited the rehydration of HMF [22]. Jain et al. used mesoporous zirconium phosphate as a catalyst for the conversion of sugars into HMF and found that mesoporous zirconium phosphate exhibited excellent catalytic activity for the production of HMF (80% yield of HMF) from fructose [23]. Despite the good catalytic activity of these catalysts, it is still necessary to overcome harsh reaction conditions and avoid rehydration of the HMF generated. Therefore, the synthesis of highly efficient and stable heterogeneous catalyst with highly selective for the conversion of fructose into HMF is very significant.
Schiff bases have been drawn attention because they contain electron-donating group (C=N), which can be coordinated with numbers of metal ions [24]. Transition metal Schiff base complexes have been widely used as homogeneous catalysts for various reactions, such as hydrogenation and Heck reaction [25, 26]. More and more studies have focused on immobilizing Schiff base complexes onto various supports to obtain different heterogeneous catalysts. The common catalyst carriers are mesoporous molecular sieves, montmorillonite, polymers, Fe3O4, carbon nanomaterials and so on.
In this paper, we first synthesize the mesoporous silica MCM-41 and then introduce aminopropyl to the surface of MCM-41. Finally, the Zr-Schiff base is combined with the modified MCM-41 to form a heterogeneous catalyst and used for the dehydration of fructose. Mesoporous silica MCM-41 and the modified mesoporous silica MCM-41 were prepared according to the literature method with a slight modification [27-29]. Zr(Ⅳ)-salen-MCM-41 preparation process was as follows: ZrOCl2·8H2O (2 mmol) was added to imine-MCM-41 (1 g) in acetonitrile (30 mL) at 80℃ under N2 atmosphere. After stirring for 6 h, the mixture was filtered and washed with water, and then dried overnight to obtain a peach powder. All the materials, characterizations used in this work and the detailed preparation methods were presented in the Supporting information.
The dehydration reaction was carried out in a 5 mL reaction vial equipped with magnetic stirrer. A typical procedure for dehydration of fructose was as follow: fructose (100 mg), catalyst (50 mg) and DMSO (2 mL) were added into the reaction vial. The reaction mixture was heated to desirable temperatures with an oil bath under strong stirring for a specific time. After the reaction, the catalyst was separated by centrifugation, then the sample was diluted with deionized water and analyzed by high-performance liquid chromatography (HPLC). The specific analysis method was shown in the Supporting information.
Infrared spectroscopy was used as a key method to characterize the functional groups of the samples. The FT-IR spectra of MCM-41, NH2-(CH2)3-MCM-41 and Zr(Ⅳ)-salen-MCM-41 were showed in Fig. S1 (Supporting information). The prominent peaks at 1089 and 802 cm-1 were assigned to asymmetric and symmetrical stretching vibration of Si-O-Si. The bands at 3428, 3401 and 3414 cm-1 in the FTIR spectra of MCM-41, NH2-(CH2)3- MCM-41 and Zr(Ⅳ)-salen-MCM-41 respectively indicated Si–OH stretching vibration. There were a symmetrical stretching vibration of –NH2 at 1565 cm-1 and a stretching vibration of –CH2 at 2934 cm-1 in curve b (Fig. S1), suggesting that the coupling agent had been successfully attached to the surface of MCM-41. The absorption peak at 1622 cm-1 could be attributed to the C¼N bond of Schiff base, indicating the formation of the Schiff base complex.
According to Fig. S2 (Supporting information), it was not difficult to find that pure MCM-41 presented spherical particles with uniform size and diameter at the nanometer level. As shown in Fig. 1a, Zr(Ⅳ)-salen-MCM-41 generated aggregation because of the incorporation of organic functional groups, but it still maintained a spherical structure. As seen from Fig. 1b, the TEM image of Zr(Ⅳ)-salen-MCM-41 confirmed that the material presented longer range order and dimensional pores, similar to the pure silicon MCM-41.

|
Download:
|
| Fig. 1. SEM image (a), TEM image (b), wide angle XRD pattern (c) of the catalyst Zr(Ⅳ)-salen-MCM-41 and low angle XRD patterns of the MCM-41 and Zr(Ⅳ)-salen-MCM-41. | |
The XRD patterns of MCM-41, Zr(Ⅳ)-salen-MCM-41 were shown in Figs. 1c and d. In the Low angle XRD pattern of pure MCM-41, a very strong reflection at 2θ = 1.89° for d (100) and two other weaker reflections at 2θ = 3.79° and 4.35° for d (110) and d (200) were observed, which could be indexed to a well-ordered mesoporous material of the hexagonal symmetry [25]. However, the characteristic reflection peaks (d (110) and d (200)) in the lowangle XRD pattern of Zr(Ⅳ)-salen-MCM-41 were significantly reduced to barely visible, which possibly due to the deterioration of MCM-41 after symmetry modification. The results provided further evidence that functionalization mainly occurred inside the mesopore channels and the catalyst remained structural ordering of the MCM-41 channels [30]. Moreover, in the wide angle XRD pattern of the Zr(Ⅳ)-salen-MCM-41, the only hump was due to the amorphous form of silica, indicating the absence of other crystalline phases.
The N2 adsorption-desorption isotherms of MCM-41 and Zr(Ⅳ)- salen-MCM-41 were shown in Fig. S3 (Supporting information). MCM-41 belonged to typical type IV adsorption isotherms with H1 hysteresis loop according to IUPAC classification, which indicated the presence of the mesoporosity [31]. On the contrary, Zr-salenMCM-41 belonged to the typical type Ⅲ adsorption isotherms according to IUPAC classification. Textural properties and acid/base sites density of the samples were summarized in Table S1 (Supporting information). MCM-41, a common mesoporous material, showed high surface areas and pore volume. Noticeably, the surface area of the Zr(Ⅳ)-salen-MCM-41 decreased a lot, which indicated that some pendant group on the surface of the catalyst had blocked the adsorption of nitrogen molecules [28]. Such significant decrease of textural properties of porous materials on grafting had been reported [32]. In addition, acid and base density of the Zr(Ⅳ)-salen-MCM-41 were determined by NH3-TPD and CO2-TPD respectively, and the results were presented in Table S1 and Fig. S4 (Supporting information). As shown in Table S1, there were a large number of acid-base sites in the catalyst, and the acid and base sites were derived from Zr4+ and aniline groups, respectively. Moreover, phenolic hydroxyl groups could also provide some basic site.
The TG curve of the synthesized materials was presented in Fig. S5 (Supporting information). The TG curve of the Zr(Ⅳ)-salen-MCM-41 material showed three individual steps of weight loss (75–150, 150– 350 and 350–600℃). A weight loss of 4.64% involved in the first step was due to the removal of physically adsorbed water. 12.45% of the weight loss was associated with the oxidative decomposition of the organic functionalgroupsin the second stage. Finally, dihydroxylation of the silicate networks resulted in a 15.25% weight loss at higher temperatures [33]. On the basis of these results, the well grafting catalyst exhibited good thermal stability below 150℃.
According to Table S2 (Supporting information), the dehydration of fructose into HMF could not occur in the absence of catalyst. Compared with SBA-15, MCM-41 had better catalytic activity for the dehydration of fructose into HMF (Table S2, entries 2, 3). The catalytic activity of metal-doped MCM-41 was higher than pure MCM-41 (Table S2, entries 4, 5) because the Lewis acid from metal could improve the catalytic activity of catalysts. Modified mesoporous materials could also promote the dehydration of fructose to a certain extent, but the effect was not significant (Table S2, entries 6, 7). On the contrary, Zr(Ⅳ)-salen-MCM-41 showed better catalytic activity for the dehydration of fructose into HMF, indicating that Zr as an active center could promote the dehydration of fructose into HMF.
The effects of reaction temperature and time for the dehydration of fructose into HMF were discussed in order to achieve maximum yield of HMF using DMSO as solvent. As shown in Fig. 2a, the yield of HMF increased as the temperature rose, achieving a maximum of 92.0% at 140℃. The main reason was that high temperature was beneficial to the dehydration of fructose, thus increasing the yield of HMF. When the reaction temperature was raised to 150℃, the yield of HMF decreased slightly because side reactions were more likely to occur at higher temperatures. Hence, 140℃ was considered as the optimum reaction temperature in subsequent experiments. The effect of reaction time was carried out at 140℃ and the results were presented in Fig. 2b. It was not difficult to find that reaction time had a great impact on the production of HMF. As the reaction time lengthened, the yield of HMF increased rapidly and reached a maximum at 4 h. Prolonging the reaction time to 5 h, the yield of HMF dropped slightly as some acidic sites were covered by the rapid formation of humus on the catalyst surface leading to a decrease in catalyst activity [34]. Based on the above analysis, 140℃ and 4 h were selected as the best reaction conditions to study the influence of other parameters on the reaction.

|
Download:
|
| Fig. 2. The effect of reaction temperature (a), reaction time (b) and different catalyst amounts (c) on the dehydration of fructose into HMF. Reaction conditions: 100 mg of fructose, 2 mL of DMSO, (a) 50 mg catalyst, 4 h; (b) 50 mg catalyst, 140℃. | |
The effect of catalyst dosages on the dehydration of fructose into HMF at 140℃ for 4 h was also investigated and the result was presented in Fig. 2c. An obvious improvement in the HMF yield was observed when catalyst dosage was increased from 30 mg to 50 mg, indicating that HMF formation was promoted by more acid sites [10]. However, the HMF yield declined slightly at high catalyst amount (60 mg). It meant that the excessive catalyst would lead to the generation of undesired products such as soluble polymers and humins [2]. Thus, 50 mg was selected as the optimal dosage of catalyst for the dehydration of fructose.
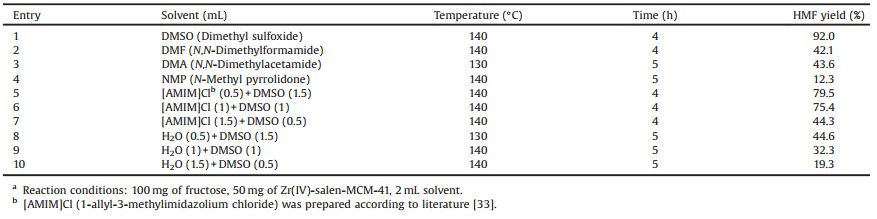
Thesolvent, animportant factor for the catalytic reaction, wasalso screened. The catalytic activity of Zr(Ⅳ)-salen-MCM-41 for the conversion offructose into HMF was evaluated in a variety of common solvents (Table 1). It was noted that the solvent showed a remarkable effect on the yield of HMF. 92.0% HMF yield was obtained in DMSO at 140℃ for 4h (Table 1, entry 1) because DMSO was favorable for the form of furanoid and suppressed side reactions [34]. However, middle-lowerHMFyield couldbe achievedwith DMA, DMFandNMP as solvent under the same conditions (Table 1, entries 2–4). In addition, the effect of biphasic system (H2O/DMSO and [AMIM]Cl/ DMSO) for the conversion of fructose into HMF was also investigated over Zr(Ⅳ)-salen-MCM-41. It was demonstrated that DMSO with the co-solvent of [AMIM]Cl in different proportions could obtain moderate HMF yield (Table 1, entries 5–7). One possible reason for this phenomenon was that the [AMIM]Cl not only acted as a solvent but also stabilized the HMF produced in the reaction mixture [35]. In order to further demonstrate that co-solvent played animportant role in the conversion of fructose into HMF, DMSO with the co-solvent of water was also used as solvent for the same reaction (Table 1, entries 8–10). However, only 19.3%–44.6% yield of HMF was detected, the results proved that a small amount of watercould lower the viscosity of reaction system, which was conducive to the hydrolysis of fructose [36]. What's more, the formed HMF combined with water could produce levulinic acid and formic acid, which would reduce the yield of HMF [37]. Thus, DMSO without co-solvent was selected as the optimum solvent in the dehydration of fructose into HMF.
|
|
Table 1 Effect of the solvents on the fructose into HMF.a |
To examine the recyclability and stability of the Zr(Ⅳ)-salenMCM-41 catalyst, a recycling experiment was performed at 140℃ for 4 h in DMSO (Fig. S6 in Supporting information). After the reaction, the catalyst was separated by filtration from the reaction mixture. The recovered catalyst was washed three times with ethanol and dried at 40℃ overnight for the next run. After four recycles, the HMF yield dropped to 79.1%, suggesting that Zr(Ⅳ)- salen-MCM-41 could be reused many times with a slight decrease in catalytic activity.
The dehydration of different substrates into HMF were further investigated over Zr(Ⅳ)-salen-MCM-41 in DMSO, and the results were summarized in Table S3 (Supporting information). 36.6% yield of HMF and 80.1% glucose conversion were obtained at 140℃ for 4 h (Table S3, entry 1). The lower selectivity of HMF was attributed to the production of fructose derived from the isomerization of glucose. Mannose and galactose were chiral isomers of glucose, so the effect was similar to glucose (Table S3, entries 2-3). In contrast, the sucrose and inulin were used as the substrates to produce the HMF with a high conversion and HMF yield (Table S3, entries 4–5), which probable reason was that these sugars were composed of fructose units and the ketose product (fructose) could be efficiently converted into HMF [4]. When the cellulose was subjected to reaction system, the HMF yield was remarkably inferior (Table S3, entry 6) because cellulose was hardly hydrolyzed in the organic media.
In summary, an inexpensive, efficient and easily regenerable heterogeneous catalyst (Zr(Ⅳ)-salen-MCM-41) was synthesized for the conversion of fructose into HMF. The results illustrated that Zr(Ⅳ)-salen-MCM-41 had higher catalytic activity for the production of HMF from fructose at 130℃ for 4 h with DMSO as solvent. Importantly, the yield of HMF decreased only 12.9% after the catalyst was reused four times. In addition, other sugars were also used as substrates for the synthesis of HMF over Zr(Ⅳ)-salenMCM-41, but the effect was not as good as fructose. Although these catalysts need to be further improved, we believe that heterogeneous catalysts similar to Zr(Ⅳ)-salen-MCM-41 will play an increasingly important role in biorefinery.
AcknowledgmentThis work was financial supported by MOE & SAFEA, 111 Project (No. B13025).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.03.026.
| [1] |
Z.Z. Yang, J. Deng, T. Pan, Q.X. Guo, Y. Fu, Green Chem. 14 (2012) 2986-2989. DOI:10.1039/c2gc35947b |
| [2] |
Q.D. Hou, W.Z. Li, M.T. Ju, et al., RSC Adv. 6 (2016) 104016-104024. DOI:10.1039/C6RA23420H |
| [3] |
P. Maki-Arvela, I.L. Simakova, T. Salmi, D.Y. Murzin, Chem. Rev. 114 (2014) 1909-1971. DOI:10.1021/cr400203v |
| [4] |
N.N. Wang, Y. Yao, W. Li, et al., RSC Adv. 4 (2014) 57164-57172. DOI:10.1039/C4RA09585E |
| [5] |
F.D. Pileidis, M.M. Titirici, ChemSusChem 9 (2016) 562-582. DOI:10.1002/cssc.201501405 |
| [6] |
Y.L. Yang, Z.T. Du, Y.Z. Huang, et al., Green Chem. 15 (2013) 1932-1940. DOI:10.1039/c3gc37133f |
| [7] |
G. Yong, Y.G. Zhang, J.Y. Ying, Angew. Chem. Int. Ed. 47 (2008) 9485-9488. |
| [8] |
B. Liu, Z.H. Zhang, ChemSusChem 47 (2016) 2015-2036. |
| [9] |
J. Wang, T. Qu, M.S. Liang, Z.B. Zhao, RSC Adv. 5 (2015) 106053-106060. DOI:10.1039/C5RA22979K |
| [10] |
T.S. Deng, J.G. Li, Q.Q. Yang, et al., RSC Adv. 6 (2016) 30160-30165. DOI:10.1039/C6RA00154H |
| [11] |
Z.H. Zhang, J.D. Zhen, B. Liu, K.L. Lv, K.J. Deng, Green Chem. 17 (2015) 1308-1317. DOI:10.1039/C4GC01833H |
| [12] |
X.W. Han, L. Geng, Y. Guo, et al., Green Chem. 18 (2016) 1597-1604. DOI:10.1039/C5GC02114F |
| [13] |
D.W. Gardner, J.J. Huo, T.C. Hoff, et al., ACS Catal. 5 (2015) 4418-4422. DOI:10.1021/acscatal.5b00888 |
| [14] |
J.B. Binder, A.V. Cefali, J.J. Blank, R.T. Raines, Energ. Environ. Sci. 3 (2010) 765-771. DOI:10.1039/b923961h |
| [15] |
H.B. Zhao, J.E. Holladay, H. Brown, Z.C. Zhang, Science 316 (2007) 1597-1600. DOI:10.1126/science.1141199 |
| [16] |
X.H. Yi, I. Delidovich, Z. Sun, et al., Catal. Sci. Technol. 5 (2015) 2496-2502. DOI:10.1039/C4CY01555J |
| [17] |
P. Zhou, Z.H. Zhang, Catal. Sci. Technol. 6 (2016) 3694-3712. DOI:10.1039/C6CY00384B |
| [18] |
R.J. van Putten, J.C. van der Waal, E. de Jong, et al., Chem. Rev. 113 (2013) 1499-1597. DOI:10.1021/cr300182k |
| [19] |
F.C. de Melo, R.F. de Souza, P.L.A. Coutinho, M.O. de Souza, J. Brazil Chem. Soc. 25 (2014) 2378-2384. |
| [20] |
A.H. Jadhav, A. Chinnappan, R.H. Patil, S.V. Kostjuk, H. Kim, Chem. Eng. J. 243 (2014) 92-98. DOI:10.1016/j.cej.2013.12.054 |
| [21] |
H.T. Han, H.Y. Zhao, Y. Liu, et al., RSC Adv. 7 (2017) 3790-3795. DOI:10.1039/C6RA26309G |
| [22] |
B. Liu, Z.Z. Gou, A.Q. Liu, Z.H. Zhang, J. Ind. Eng. Chem. 21 (2015) 338-339. DOI:10.1016/j.jiec.2014.02.043 |
| [23] |
A. Jain, A.M. Shore, S.C. Jonnalagadda, K.V. Ramanujachary, A. Mugweru, Appl. Catal. A:Gener. 489 (2015) 72-76. DOI:10.1016/j.apcata.2014.10.020 |
| [24] |
S. Narang, R. Mehta, S.N. Upadhyay, Ind. Eng. Chem. Res. 52 (2013) 3967-3973. DOI:10.1021/ie3033697 |
| [25] |
C.G. Arellano, A. Corma, M. Iglesias, F. Sánchez, Eur. J. Inorg. Chem. 7 (2008) 1107-1115. |
| [26] |
C.G. Arellano, A. Corma, M. Iglesias, F. Sánchez, Adv. Synth. Catal. 346 (2004) 1758-1764. DOI:10.1002/adsc.200404119 |
| [27] |
A. Jafarzadeh, S. Sohrabnezhad, M.A. Zanjanchi, M. Arvand, Micropor. Mesopor. Mat. 236 (2016) 109-119. DOI:10.1016/j.micromeso.2016.08.035 |
| [28] |
X. Yuan, G.F. Shan, L.X. Li, J. Wu, H.A. Luo, Catal. Lett. 145 (2015) 868-874. DOI:10.1007/s10562-015-1499-2 |
| [29] |
M. Hajjami, F. Ghorbani, S. Roshani, S. Rahimipanah, J. Porous. Mat. 23 (2016) 689-699. DOI:10.1007/s10934-016-0124-0 |
| [30] |
M. Hajjami, F. Ghorbani, S. Roshani, S. Rahimipanah, Chin. J. Catal. 36 (2015) 1852-1860. DOI:10.1016/S1872-2067(15)60968-8 |
| [31] |
M. Fadhli, I. Khedher, J.M. Fraile, J. Mol. Catal. A:Chem. 420 (2016) 282-289. DOI:10.1016/j.molcata.2016.05.001 |
| [32] |
M. Hajjami, Z. Yousofvand, Catal. Lett. 145 (2015) 1733-1740. DOI:10.1007/s10562-015-1572-x |
| [33] |
Y.Y. Wang, L.H. Zhang, D.D. Wang, X.Y. Guo, S.H. Wu, J. Chromatogr. A 1478 (2016) 26-34. DOI:10.1016/j.chroma.2016.11.028 |
| [34] |
Z. Zhang, B. Du, L.J. Zhang, et al., RSC Adv. 3 (2013) 9201-9205. DOI:10.1039/c3ra41912f |
| [35] |
Z. Zhang, B. Du, Z.J. Quan, Y.X. Da, X.C. Wang, Catal. Sci. Technol. 4 (2014) 633-638. DOI:10.1039/c3cy00888f |
| [36] |
T.Y. Cheng, P.Y. Chao, Y.H. Huang, et al., Micropor. Mesopor. Mat. 233 (2016) 148-153. DOI:10.1016/j.micromeso.2016.01.015 |
| [37] |
C.H. Song, H. Liu, Y. Li, et al., Chin. J. Chem. 32 (2014) 434-442. DOI:10.1002/cjoc.201400054 |
 2019, Vol. 30
2019, Vol. 30