b Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education, and Key Lab of Visual Damage and Regeneration & Restoration of Chongqing, Bioengineering College, Chongqing University, Chongqing 400030, China;
c Chongqing Engineering and Technology Research Center of Intelligent Rehabilitation and Eldercare, Chongqing City Management College, Chongqing 401331, China
Liposomes are synthetic lipid vesicles with a similar membrane structure as the cell membrane, and those with a diameter larger than 1 mm are specifically called as giant liposomes (vesicles) [1-3]. Giant liposomes have obtained widespread attention for their potential application as drug delivery vehicles, microreactors, and model systems of cell membranes. After the electroformation method of giant liposomes was first reported by Angelova and Dimitrov [4], many devices and methods were proposed to improve it [2, 4-28].
Some devices consisted of a pair of parallel electrode wires in a chamber [4, 5, 8-13], and lipid film was deposited on the wires for the electroformation of giant liposomes. Their structures were very simple and easy for fabrication. However, the wires had a small surface area for lipid film deposition, and they were also opaque and hard to be observed.
Another type of electroformation devices frequently used a spacer between two ITO (indium tin oxide) glass slides to build reactor chambers. Lipid film was deposited onto the conductive ITO glass slides [2, 14-22], which provided a larger surface area and were transparent for microscopic observation, so this kind of device was more widely used. Some other methods were also developed by using different lipid film formation techniques [18-21] or changing the solutions components and ionic strength [17, 22].
Devices with more complex structures and more functions were developed based on the previous studies. For example, sandwiched microchannels were fabricated between electrodes [23, 24]. Nonconductive substrates were used to deposit lipid film between electroformation electrodes [25, 29]. An array of holes were built on silicon electrodes [27, 28] to organize the lipid film and concentrate the electric field, thereby controlling the size distribution of formed giant liposomes. Coplanar interdigitated ITO electrodes [6] or symmetrical comb teeth coplanar electrode arrays [7] were also used to control the liposome formation.
However, up until now, the existing devices and methods still had some disadvantages, such as low preparation efficiency, poor repeatability, complex, costly, and so on. Numerous factors may affect the electroformation, so multiparameter analysis is preferred to achieve a suitable formation condition, but it is fussy in traditional designs. Furthermore, in most electroformation devices, the collection of giant liposomes is unsatisfactory. Mostly, it depends on an extra extraction step in another device, during which a large number of giant liposomes with fragile membranes will be broken, and it is also difficult to obtain liposomes of desired sizes or enclosing required substances.
In this study, an integrated microchip was designed for liposome electroformation and collection. Simple and easy to use were emphatically considered here. Multiple reactor chambers and parallel manipulation were chosen to simultaneously study different impact factors on the electroformation. An integrated onchip collection module was another pursuit.
The microchip was composed of three layers (Fig. 1). The bottom layer was a circular ITO glass substrate, on which two polydimethylsiloxane (PDMS) layers were bonded. Reactor chambers, collection chambers, waste chambers and microchannels were fabricated on these PDMS layers (Fig. 1C). On the middle PDMS layer, sixteen circular reactor chambers (6 mm diameter) with two radial microchannels (800 μm width) evenly distributed at the edge. At the corresponding locations of the upper PDMS layer, there were sixteen rectangular cavities (10 mm × 10 mm × 2 mm), which were used to fix rectangular ITO glass cover slides (10 mm × 6 mm × 1 mm). At the center of the middle PDMS layer, there was a large waste chamber (25 mm diameter) surrounded by four small waste chambers (800 μm diameter, a part of them located on the upper PDMS layer). Four individual microchannels connected with the large and small waste chamber. On the upper layer and just over the large waste chamber on the middle layer, there was a collection chamber (30 mm diameter), which connected with the sixteen rectangular chambers on the same layer through individual microchannels (800 μm width). Used as a filter, a Nuclepore track-etch membrane (10.0 μm pore size, circles, 47 mm, 100/pk) was sandwiched between the collection chamber and the large waste chamber. After giant liposomes were formed in the reactor chambers, fresh buffer was injected to drive them to the collection chamber. Waste liquid flowed through the filter to the large waste chamber, and pumped away from the four small waste chambers. On this microchip, ITO glass slides (10 Ω/sq surface resistance) served as electrodes and support. PDMS layers were used to form the sidewall of different chambers and microfluidic channels. The detailed fabrication method of this chip is described in Supporting information (Fig. S1).

|
Download:
|
| Fig. 1. An integrated microchip for liposome electroformation and collection. (A) Chip prototype, (B) three-dimensional chip structure, and (C) assembly drawing. | |
In the electroformation, many factors such as the composition of lipid solution and buffer solution, lipid film thickness, electrode properties, as well as the strength, frequency and duration of the electric field [5, 8, 11, 15, 20, 23, 25, 27, 30], may influence the preparation effect of giant liposomes. For a better preparation effect or some special applications, a lipid mixture is usually used. The composition of the mixture may influence the mobility and stability of the formed liposome membrane. Cholesterol is a commonly used component in the mixture, and it has been verified to have the capability to increase the stiffness of lipid membranes, although a high ratio of cholesterol may disturb the formation of liposomes (e.g., 3β-hydroxy-5-cholestene (cholesterol):L-α-phosphatidylcholine (PC) > 1:6) [11]. The surface property of the substrate, the concentration and volume of the lipid solution, and the loading style determine the topography of the lipid film deposited on the substrate [31, 32]. A too thick or too thin lipid film is not suitable for the formation of giant unilamellar liposomes. Electric fields affect liposome formation by some mechanisms such as electrostatic interaction. Redistribution of counter ions on the lipid membrane may decrease the surface tension and electrokinetic effects, and thus influence the formation. All these impacts may be different for whether there are electric fields or not. Thus, rapid multiparameter analysis is necessary to achieve an optimal value in a new electroformation study.
Since so many factors were required to be screened, microchip with multiple reactor chambers has prominent advantages. In this study, some influencing factors were divided into 2 groups to carry out orthogonal experiments with three factors and four levels, assuming that there was no interrelationship between them. In the first group, the three factors were the mass ratio, concentration and volume of the lipid solution. In the second group, they were the electric voltage, frequency and the exertion time.
PC and cholesterol were used as the lipid samples for electroformation. Based on a previous study [33], the lipid mixture (PC and cholesterol) and fluorescent dyes DiO (3, 30-dioctadecyloxacarbocyanine perchlorate, ex/em: 484/501 nm, molecular probes) was dissolved in ether to form lipid solutions with different concentrations for orthogonal experiments. Lipid solutions with distinct mass ratios (cholesterol:PC = 1:1, 1:3, 1:5, 1:7), different PC concentrations (0.5, 3, 6, 9 mg/mL) and different volumes (2, 4, 6, 8 mL) were investigated. To study the impact of multiple parameters, 16 experiments were carried out in 16 individual reactor chambers (Fig. 1) simultaneously, and all experiments were repeated at least 3 times.
Before the electroformation experiments, lipid solution was dropped onto the bottom of each reactor chamber and stored under reduced pressure for 2 h to form lipid film. Then, each reactor chamber was covered by a small ITO glass slide and filled with deionized water (57 μL) from the connected microchannels. An AC electric signal (sinusoidal wave, 8 V peak-to-peak, 10 Hz) was subsequently loaded on the ITO glass slides for 3 h (Figs. 2A–E), and the formation process of giant liposomes was observed by using a phase contrast inverted fluorescence microscope (DMI4000 B, Leica, Germany) or a laser scanning confocal microscope (A1R+, Nikon, Japan). In order to accurately count the formed liposomes, the reactor chamber was divided into four regions by two mutually perpendicular diameters. Spherical giant unilamellar liposomes in different planes were photographed by using a microscopic camera system and counted. Finally, the total number of giant liposomes in these four regions is summed up. All results were analyzed by using PASW Statistics 18 software (IBM, America).
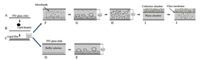
|
Download:
|
| Fig. 2. The process of giant liposome electroformation and collection on the microchip. (A) Clean the ITO glass slide, (B) drip lipid solution, (C) form lipid film, (D) cover ITO glass slide and add buffer solution, (E) apply electric field, (F) cover ITO glass slide and add microbead suspension, (G) apply electric field, (H) form giant liposomes, (I) load buffer solution to drive the formed liposomes into the collection chamber, and (J) trap desired giant liposomes on the filter membrane. | |
From the results, when the ratio of cholesterol increased, a higher yield of giant liposomes and a higher ratio of spherical giant unilamellar liposomes, which is desired in most applications, were achieved. However, when the ratio of cholesterol to PC was higher than 1:5, the yields of both giant liposomes and spherical giant unilamellar liposomes decreased. These results verified that the composition of lipid solutions might influence the formation of giant liposomes. The probable mechanism is that cholesterols can adjust the mobility of the membrane and increase its stiffness by combining with PCs to keep them from forming crystal structures. Thus, a lower ratio of cholesterol may cause deficient membrane stability, and the resulting giant liposomes are easily breakable. However, membrane mobility at a higher ratio of cholesterol is too scanty to form giant liposomes.
The yield of giant liposomes increased with the increase of the PC concentration (Fig. 3). However, when the PC concentration exceeded a threshold value (6 mg/mL), more than 50% of the formed giant liposomes were multilamellar, stacked and nonspherical. The ratio of spherical giant unilamellar liposomes, which could be determined by using a laser scanning confocal microscope (Fig. 3E), rapidly decreased. When a lipid solution droplet was dropped onto the chip, it spread and formed a special pattern on the bottom surface under the effect of gravity and surface tension. During its rapid spreading, the solvent rapidly volatilized and then the solved lipid molecules formed multiple ring-shaped lipid films of distinct thicknesses on the bottom surface [32]. Thicker film had more lipid layers, and only lipid film of suitable thickness could form giant unilamellar liposomes [5, 7]. For thicker lipid film, more lipid layers could be peeled off to form giant liposomes. However, if the lipid film was too thick, the separation of lipid layers from it was difficult, and only a few giant multilamellar liposomes could be formed.

|
Download:
|
| Fig. 3. Giant liposomes formed in different PC concentrations. (A) 0.5 mg/mL, (B) 3 mg/mL, (C) 6 mg/mL, (D) 9 mg/mL. (E) A spherical giant unilamellar liposome under a laser scanning confocal microscope. All scale bars are 10 μm. | |
According to the experimental results (showed in Tables S1 and S2 in Supporting information), the impacts of the three factors were ranked as concentration > distinct mass ratio > volume of lipid solution. Based on the single-factor estimate and pairwise comparison, the appropriate mass ratio was chosen as cholesterol: PC = 1:5, the optimal concentration of PC and cholesterol solution was 3 mg/mL and 0.6 mg/mL, respectively, and the optimal lipid solution volume was 2 μL (Fig. 4A). In this case, steady and spherical unilamellar giant liposomes, without other smaller encapsulated liposomes, were formed.

|
Download:
|
| Fig. 4. Results of the orthogonal experiments with three factors at four levels. (A) Three factors were the mass ratio, concentration, and volume of the lipid solution. (B) The factors were the electric voltage and exertion time. Estimated marginal means was the result of the number of formed spherical giant unilamellar liposomes. | |
Besides the main influencing factors of lipid solution, three electric signal parameters, including the electric voltage, frequency and exertion time, were also investigated for their impacts on the electroformation. Concentrations of PC and cholesterol were fixed at 3 mg/mL and 0.6 mg/mL, respectively, and volumes of the lipid and buffer solution were 2 μL and 57 μL, respectively. Three factors and four levels of different electric signals were chosen as voltages (1, 4, 8, 13 V), electric-field frequencies (2, 10, 500, 1000 Hz), and exertion time (1, 3, 5, 7 h). The experimental results were shown in Tables S3 and S4 (Supporting information). Their influences could be ranked as exertion time > voltage > frequency. There were significant differences among different exertion times and voltages, but almost no differences for different frequencies.
AC electric fields are commonly loaded in electroformation to exert pressure and produce vibration on the lipid film and urge its swelling, bending, separating and sealing. Theycan also enhance the hydration, osmosis and electrostatic effect. Among the main electric parameters, the exertion time directly influences the electroformation processes, and too short exertion time cannot achieve a good effect. However, if the exertion time is long enough, further prolonging it does not obviously increase the yield of giant liposomes.
Commonly, increasing the electric voltage (electric-field strength) will obtain a higher formation velocity and larger giant liposomes. When the electric voltage increased to 13 V, formed giant liposomes gradually fused with each other to form larger giant liposomes (> 100 μm). These liposomes were not spherical and stable, and it was difficult to collect them.
Although the influence of frequency on liposome formation was not significant, some differences of distinct frequencies were also observed. For higher frequencies (1000 Hz and 500 Hz), formed giant liposomes were more uniform, but lipid film swelled slowly, especially at the thicker region. As the frequency reduced to 10 Hz, the lipid film swelled more quickly, and the thicker lipid film could also swell and form more giant liposomes. When the frequency decreased to 2 Hz, some huge giant liposomes (> 200 μm) were formed, especially at the thinner lipid film, but they were fragile and the collection was difficult. One possible reason is that the electric frequency may affect the vibration frequency of the lipid film, and lower vibration frequency accelerates the swelling of the lipid film and helps the electroformation of giant liposomes. Based on the single-factor estimate and pairwise comparison (Fig. 4B), the optimized exertion time, voltage and frequency were chosen as 5 h, 4 V and 10 Hz, respectively.
Experimental results showed that the electroformation of giant liposomes on the microchip was influenced by many factors, such as the compositions of the lipid mixture, the thickness of lipid film, as well as the voltage, exertion time and frequency of the applied electric field. While the exact mechanism of the electroformation of giant liposomes is still unclear [11, 15, 34, 35], numerous studies have been done to explore the influences of these factors. For example, Angelova et al. [5] put forward an electroswelling mechanism based on the electroosmotic effect, osmotic pressure, membrane elasticity, hydration, and other factors such as van der Waals force. They suggested that the formation of liposomes required the separation and bending of lipid films. Normal and tangential forces on lipid films were involved in the electroformation process to let the small unilamellar vesicles grow into giant liposomes, and the electric field could change both types of forces. Shimanouchi et al. [30] suggested that temperature, lipid composition and membrane fluidity were the dominant factors in the formation of giant liposomes. Horger [36] combined the previous viewpoints and approved that the electroformation process could be separated into three stages [4, 5, 14, 37, 38]. In the first stage, lipids in a solid lipid film were hydrated to induce the self-assembly and separation of lipid lamellae [4, 38]. The second stage involved swelling of liposomes due to normal forces on the bilayers [5, 37], which were provided by osmotic pressure [5], line tension [5] and electric fields [5, 24], and the recruitment of lipids from the lipid film [4, 23]. The final stage was the fusion of adjacent liposomes due to mechanical stresses [14, 15]. However, these theories could not solve the diverse and complex problems in practical studies. Thus, pursuing an optimal condition for highefficiency electroformation is nevertheless dependent on multiparameter analyses.
In this study, the experimental results partly accorded with Shimanouchi's theory and Horger's view, i.e., properties of lipids affected electroformation in the first stage. However, this effect mainly appeared in a low concentration of lipid solution to affect the quantity and position of formed giant liposomes. On the other hand, these results also partly accorded with Angelova's opinion and Horger's view, i.e., an electric field obviously affected electroformation in the second stage. Driven by the electric field-induced force, giant liposomes could form in some highly inhibited conditions, such as lipid solution with an abnormal ratio of cholesterol (e.g., cholesterol:PC > 1:6) [11] and thicker lipid films. In this case, the electric field played a more important role than lipid properties. Small liposomes would gradually swell, and some contiguous liposomes fused into bigger ones. A higher electric voltage accelerated the expansion, and a lower frequency helped the swelling of lipid film and the formation of giant liposomes.
In order to realize collection of liposomes on the same chip, a circular collection chamber and several waste chambers were also integrated on it. A Nuclepore track-etch membrane was tailored and sandwiched between the collection chamber and the large waste chamber as a filter. After the giant liposomes had been electroformed on the chip, buffer solution was added from the surrounding reactor chambers at different flow rates (from 50 μL/h to 245μL/h) to flush the formed liposomes to the collection chamber. The liquid diffused through the membrane was removed from the surrounding waste chambers at different rates (from 10 μL/h to 100 μL/h). Experimental resulted showed that higher pumping rates (50–102 μL/h) of buffer and lower extracting rates (10–60 μL/h) of waste liquid caused less damage to the giant liposomes. Thus, 76 μL/h and 30 μL/h were selected as the optimal pumping rate and extracting rate, respectively.
One of the main applications of giant liposomes is used as micro-scale carriers. Here microbeads with 2 μm diameter (specification range 1.80–2.20 μm) were used as samples, which were encapsulated by liposomes, to test the electroformation of giant liposome carriers. Desired giant liposomes size was set as larger than ten microns, and experimental parameters were chosen according to the above results. After the formation of a lipid film, microbeads suspension (2 ×106 /mL) was loaded into each reactor chamber. Giant liposomes enclosing microbeads were electroformed after loading the electric signal for 5 h (Fig. 5A). Then, 80 μL buffer solution was loaded into each reactor chamber by an injection pump to drive the flow of formed liposomes (Fig. 5B) into the collection chamber. Desired giant liposomes would be trapped on the filter membrane (Fig. 5C) because their sizes were larger than the pores in the membrane (10 μm). Other components within the solution, such as unencapsulated beads, lipid clusters and small liposomes (diameter < 10 μm), flowed through the membrane to the waste chamber (Figs. 2A–C and F–J). The collection ratio of the giant liposomes with encapsulating microbeads was approximately 40%.
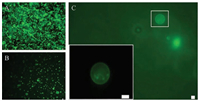
|
Download:
|
| Fig. 5. Giant liposomes enclosing microbeads. (A) Formed liposomes in the reactor chamber. (B) Flow of formed liposomes. (C) One liposome in the collection chamber (high magnification of the white box is shown at the lower left corner). All scale bars are 10 μm. | |
To the best of our knowledge, liposomes with desired sizes are often screened by centrifugation, and liposomes that enclose required substances are often purified by dialysis or centrifugation [39]. However, almost no studies could be found on effectively collecting giant liposomes carriers of the desired sizes. Furthermore, the dialysis method is time-consuming and requires a large volume of solution, which is not specific to the size-based screening. The centrifugation method has a low collection rate for giant liposomes, in which some giant liposomes are broken or deformed, and the surrounding substances also affect the giant liposomes during centrifugation [40]. We propose a new method in this study, which can achieve simultaneous collection from multiple reactor chambers. By combining with the filter membrane, giant liposomes with desired sizes can be selected according to the pore size.
A microchip-based approach has been presented in this study for the electroformation and collection of giant liposomes. Several different parameters have been investigated for their impacts on liposome electroformation, including the mass ratio, concentrations of PC and cholesterol, lipid solution volume, and electric field. Concentrations of PC and cholesterol as well as the lipid solution volume affected the quantity and position of the formed giant liposomes. According to the experiments, optimal concentrations of PC and cholesterol were chosen as 3 mg/mL and 0.6 mg/mL, respectively, and the optimal volume of the lipid solution was 2 μL. The electric field could obviously affect liposome formation. It helped the formation of liposomes in some highly inhibited conditions by accelerating their expansion and fusion with adjacent liposomes, as well as controlling the sizes of giant liposomes. The optimal voltage, frequency and exertion time of the sinusoidal electric signal were 4 V, 10 Hz and 5 h, respectively. Using these optimal parameters, steady and spherical giant unilamellar liposomes without other smaller liposomes encapsulated were formed. Giant unilamellar liposomes with desired properties could be collected on the same chip.
By using a simple fabrication process, a reactor chamber array and a collection chamber could be integrated on the same chip. Multiple parameters can be tested simultaneously and efficiently, and a large number of giant unilamellar liposomes can be rapidly electroformed and collected. Thus, this new chip may become an effective tool in the research of giant unilamellar liposomes and giant liposomes carriers.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 81501617, 81871450, 21827812), Science and Technology Planning Project of Yuzhong District, Chongqing, China (No. 20170122), the Program of International S & T Cooperation (No. 2014DFG31380), and the Foundation for Higher Education Young Key Teacher of Chongqing, China.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.12.001.
| [1] |
A. Jesorka, O. Orwar, Annu. Rev. Anal. Chem. 1 (2008) 801-832. DOI:10.1146/annurev.anchem.1.031207.112747 |
| [2] |
K. Kuribayashi, G. Tresset, P. Coquet, H. Fujita, S. Takeuchi, Meas. Sci. Technol. 17 (2006) 3121-3126. DOI:10.1088/0957-0233/17/12/S01 |
| [3] |
P. Walde, K. Cosentino, H. Engel, P. Stano, ChemBioChem 11 (2010) 848-865. DOI:10.1002/cbic.201000010 |
| [4] |
M.I. Angelova, D.S. Dimitrov, Faraday Discuss. Chem. Soc. 81 (1986) 303. DOI:10.1039/dc9868100303 |
| [5] |
M.I. Angelova, D.S. Dimitrov, A mechanism of liposome electroformation, in: V. Degiorgio (Ed.), Trends in Colloid and Interface Science Ⅱ, Springer-Verlag, New York, 1988, pp. 59-67.
|
| [6] |
H. Bi, B. Yang, L. Wang, W. Cao, X. Han, J. Mater. Chem. A 1 (2013) 7125-7130. DOI:10.1039/c3ta10323d |
| [7] |
Q. Wang, X. Zhang, T. Fan, et al., Micromachines 8 (2017) 24. DOI:10.3390/mi8010024 |
| [8] |
D.S. Dimitrov, M.I. Angelova, Prog. Colloid Polym. Sci. 73 (1987) 48-56. DOI:10.1007/3-798-50724-4 |
| [9] |
R. Wick, M.I. Angelova, P. Walde, P.L. Luisi, Chem. Biol. 3 (1996) 105-111. DOI:10.1016/S1074-5521(96)90286-0 |
| [10] |
P. Bucher, A. Fischer, P.L. Luisi, T. Oberholzer, P. Walde, Langmuir 14 (1998) 2712-2721. DOI:10.1021/la971318g |
| [11] |
L. RuthMontes, A. Alonso, F.M. Goñi, L.A. Bagatolli, Biophys. J. 93 (2007) 3548-3554. DOI:10.1529/biophysj.107.116228 |
| [12] |
T. Pott, H. Bouvrais, P. Méléard, Chem. Phys. Lipids 154 (2008) 115-119. DOI:10.1016/j.chemphyslip.2008.03.008 |
| [13] |
V. Pereno, D. Carugo, L. Bau, et al., ACS Omega 2 (2017) 994-1002. DOI:10.1021/acsomega.6b00395 |
| [14] |
M.I. Angelova, S. Soléau, P. Méléard, F. Faucon, P. Bothorel, Preparation of giant vesicles by external AC electric fields. Kinetics and applications, in: C. Helm, M. Lösche, H. Möhwald (Eds.), Trends in Colloid and Interface Science VI, Springer-Verlag, New York, 1992, pp. 127-131.
|
| [15] |
L. Mathivet, S. Cribier, P.F. Devaux, Biophys. J. 70 (1996) 1112-1121. DOI:10.1016/S0006-3495(96)79693-5 |
| [16] |
Y.M.S. Micheletto, C.M. Marques, Silveira Nd.Pd., A.P. Schroder, Langmuir 32 (2016) 8123-8130. DOI:10.1021/acs.langmuir.6b01679 |
| [17] |
M.D. Collins, S.E. Gordon, J. Vis. Exp. (2013) 50227. |
| [18] |
P. Taylor, C. Xu, P.D.I. Fletcher, V.N. Paunov, Chem. Commun. (2003) 1732. |
| [19] |
P. Taylor, C. Xu, P.D.I. Fletcher, V.N. Paunov, Phys. Chem. Chem. Phys. 5 (2003) 4918-4922. DOI:10.1039/b308082j |
| [20] |
D.J. Estes, M. Mayer, Colloid Surf. B 42 (2005) 115-123. DOI:10.1016/j.colsurfb.2005.01.016 |
| [21] |
T.J. Politano, V.E. Froude, B. Jing, Y. Zhu, Colloid Surf. B 79 (2010) 75-82. DOI:10.1016/j.colsurfb.2010.03.032 |
| [22] |
Q. Li, X. Wang, S. Ma, Y. Zhang, X. Han, Colloid Surf. B 147 (2016) 368-375. DOI:10.1016/j.colsurfb.2016.08.018 |
| [23] |
D.J. Estes, M. Mayer, BBA-Biomembranes 1712 (2005) 152-160. DOI:10.1016/j.bbamem.2005.03.012 |
| [24] |
D.J. Estes, S.R. Lopez, A.O. Fuller, M. Mayer, Biophys. J. 91 (2006) 233-243. DOI:10.1529/biophysj.105.076398 |
| [25] |
Y. Okumura, H. Zhang, T. Sugiyama, Y. Iwata, J. Am. Chem. Soc. 129 (2007) 1490-1491. DOI:10.1021/ja068127x |
| [26] |
Y. Okumura, Y. Iwata, Membranes 1 (2011) 109-118. DOI:10.3390/membranes1020109 |
| [27] |
M.L. Berre, A. Yamada, L. Reck, Y. Chen, D. Baigl, Langmuir 24 (2008) 2643-2649. DOI:10.1021/la703391q |
| [28] |
A. Diguet, M.L. Berre, Y. Chen, D. Baigl, Small. 5 (2009) 1661-1666. DOI:10.1002/smll.v5:14 |
| [29] |
Y. Okumura, T. Sugiyama, Membranes 1 (2011) 184-194. DOI:10.3390/membranes1030184 |
| [30] |
T. Shimanouchi, H. Umakoshi, R. Kuboi, Langmuir 25 (2009) 4835-4840. DOI:10.1021/la8040488 |
| [31] |
T.L. Marin, BostonThe Proceedings of the COMSOL Users Conference2006, The Proceedings of the COMSOL Users Conference (2006) 1-8.
|
| [32] |
R.D. Deegan, O. Bakajin, T.F. Dupont, et al., Nature 389 (1997) 827-829. DOI:10.1038/39827 |
| [33] |
Z. Wang, N. Hu, L.H. Yeh, et al., Colloid Surf. B 110 (2013) 81-87. DOI:10.1016/j.colsurfb.2013.04.042 |
| [34] |
D. Constantin, C. Ollinger, M. Vogel, T. Salditt, Eur. Phys. J. E 18 (2005) 273-278. DOI:10.1140/epje/e2005-00028-7 |
| [35] |
J. Leng, S.U. Egelhaaf, M.E. Cates, Europhys. Lett. 59 (2002) 311-317. DOI:10.1209/epl/i2002-00243-1 |
| [36] |
K.S. Horger, Formation of Physiologically Relevant Liposomes, the University of Michigan, 2013, pp. 20-22.
|
| [37] |
F.M. Menger, M.I. Angelova, Acc. Chem. Res. 31 (1998) 789-797. DOI:10.1021/ar970103v |
| [38] |
Y. Yamashita, M. Oka, T. Tanaka, M. Yamazaki, Biochim. Biophys. Acta 1561 (2002) 129-134. DOI:10.1016/S0005-2736(02)00338-3 |
| [39] |
S.M. Nomura, S. Kondoh, W. Asayama, et al., J. Biotechnol. 133 (2008) 190-195. DOI:10.1016/j.jbiotec.2007.08.023 |
| [40] |
K. Shigyou, K.H. Nagai, T. Hamada, Langmuir 32 (2016) 13771-13777. DOI:10.1021/acs.langmuir.6b02448 |
 2019, Vol. 30
2019, Vol. 30 

