b Beijing National Laboratory of Molecular Sciences, Key Laboratory of Analytical Chemistry for Living Biosystems, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China;
c University of Chinese Academy of Sciences, Beijing 100049, China;
d National Center for Children's Health, Beijing Children's Hospital, Beijing 100045, China
β-Lactam antibiotics are a class of broad-spectrum antibiotics, which contain a β-lactam ring in their molecular structures, and are widely used in antibacterial infections with the advantages of strong antibacterial activity and wide indications [1]. Among these β-lactam antibiotics, cephradine and cephalexin are stable to β-lactamase, which also possess a strong sterilization effect to many broad-spectrum antibiotic resistant bacterium [2]. Amoxicillin is a kind of penicillin with the ability to sterilize and penetrate cell walls and has showed extensive applications in many ways [3]. In recent years, many kinds of β-lactam antibiotics have been used in clinic treatment for antibacterial infections in hospitals. However, low concentration of β-lactam antibiotics in human body may lead to a decrease of the therapeutic efficacy, while high concentration of the drugs may induce organ dysfunction [4]. Therefore, it is necessary and important to analyze the β-lactam antibiotics in human body fluids.
In recent decades, spectroscopy, voltammetry, microbial assay, high performance liquid chromatography and capillary electrophoresis have been widely used for determination of β-lactam antibiotics [5]. Among these methods, micellar electrokinetic chromatography (MEKC) is preferential for neutral compounds separation and has been developed for analysis of β-lactam antibiotics due to its advantages in high separation efficiency, short separation time and low amount of samples [6-9]. For examples, Serrano and co-workers [8] have successfully separated β-lactam antibiotics in waste water samples by MEKC with 15.0 mmol/L sodium dodecylsulphate in electrophoresis running buffer. Michalska and colleagues [9] have used MEKC technique to separate carbapenems with 180.0 mmol/L sodium dodecylsulphate in running buffer. However, addition of substantial surfactants in running buffer may interfere with the stability of MEKC system [10]. Moreover, once unknown neutral drugs are analyzed by capillary electrophoresis-mass spectrometry protocol, the surfactants in MEKC running buffer will induce the contamination of the ion source in mass spectrometry system and suppress the signals of the analytes [11, 12], which will greatly limit the application of the protocol. Therefore, it is urgent to develop a new capillary electrophoresis method for determination and analysis of β-lactam antibiotics without surfactants in running buffer.
Open-tubular capillary electrochromatography (OT-CEC) features simple preparation, high separation efficiency and high selectivity. Interestingly, multifunctional capillaries in OT-CEC system could be realized by immobilizing different materials on the surface of the capillary through covalent bonding, physical absorption and layer-by-layer assembling. Importantly, the OT-CEC technique by immobilizing surfactants or pseudo surfactants on the wall of the capillary could be used to separate neutral drugs without adding surfactants in running buffer and without contaminating the ion source in capillary electrophoresis-mass spectrometry system. Although Shen et al. [13] successfully separated several steroids by OT-CEC protocol with polymer based nonionic surfactants as the coating, rare OT-CEC methods for β-lactam antibiotics separation have been reported until now. Thus, it is meaningful and desirable to explore novel OT-CEC systems by making new stationary phase on the capillary surface for analysis and separation of β-lactam antibiotics.
Polymer-based coatings, which could act as surfactants or pseudo surfactants in OT-CEC systems [14], have been setup owing to its multiple functionalized active sites, various polymerization methods, and good stability. Poly(maleic anhydridestyrene) (P(MAn-St)) is a kind of amphiphilic polymer and can form a uniform coating on the inner wall of the capillaries. Meanwhile, as a typical temperature responsive polymer, poly(N-isopropylacrylamide) (PNIPAm) can tune its hydrophobic/hydrophilic property with the temperature changes. Therefore, it is presumed that a new block co-polymer based P(MAn-St-NIPAm) should be synthesized. Moreover, by taking its advantages in amphiphilic and temperature sensing properties, a unique block copolymer coating could be made and used in OT-CEC system for separation of β-lactam antibiotics.
In this work, a new OT-CEC method was constructed with P(MAn-St-NIPAm) as the coating for separation of β-lactam antibiotics, including cephradine, cephalexin and amoxicillin (Fig. S1 in Supporting information). The block co-polymer was prepared through reversible addition fragmentation chain transfer (RAFT) polymerization method and immobilized in the wall of the capillary. Three β-lactam antibiotics were baseline separated by the developed OT-CEC protocol. Finally, it was successfully applied in the analysis of β-lactam antibiotics in human serums.
The block co-polymer P(MAn-St-NIPAm) was synthesized through RAFT reaction as shown in Fig. S2 in Supporting information. First, the macromolecular transfer agent P(MAn-St) was synthesized, and then P(MAn-St-NIPAm) was prepared through reinitiate method with P(MAn-St) as the chain transfer agent. The molecular weight of the P(MAn-St-NIPAm) was 40.7 kD characterized by gel permeation chromatography.
Fourier transform infrared spectroscopy measurement was carried out to verify the successfully synthesis of the block copolymer P(MAn-St-NIPAm). As displayed in Fig. S3a in Supporting information, the classical symmetric and antisymmetric stretching vibration absorption peak of C=O bond in maleic anhydride were observed at 1857.4 cm-1 and 1778.3 cm-1. The stretching vibration absorption of CH2 in benzene ring was found at 2923.9 cm-1 and C=C was found at 1452.3 cm-1, respectively. The results reflected that the P(MAn-St) was synthesized successfully. Fig. S3b in Supporting information depicts that besides the absorption peaks in P(MAn-St), the stretching vibration absorption peak of O—H in amino amide at 1650.9 cm-1 and bending vibration absorption of N—H at 1537.2 cm-1 were observed. Moreover, the stretching vibration absorption of N—H at 3064.7 cm-1 and 2931.7 cm-1 were also obtained. Fig. S4 in Supporting information shows the 1H NMR characteristic proton signals of phenyl groups in PSt at δ 6.3–7.5, and the methane proton signal of the isopropyl group in PNIPAm at δ 3.9 could be observed. These results indicated the block copolymer P(MAn-St-NIPAm) was successfully prepared.
Bare capillary was first activated with NaOH, and then modified with APTES. Finally the anhydride bond of the polymer chemically would bond with -NH2 on the modified capillary. Coating process was shown in Fig. S5 in Supporting information. SEM was used to investigate the surface morphologies of bare (Fig. S6a in Supporting information) and coated capillary (Fig. S6b in Supporting information). Comparing with the bare capillary, a thin layer of polymer coating could be found on the surface of the coated capillary, which was significantly different from the smooth surface of bare one. It demonstrated the successful immobilization of co-polymer onto the capillary inner wall.
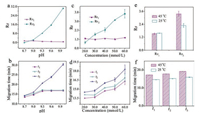
Effect of running buffer pH and concentrations, and the organic additives on OT-CEC separation efficiency has been investigated in detail. Considering the pKa values of the three selected analytes (cephradine, cephalexin and amoxicillin) and the MEKC conditions described in reference [9], running buffer pH range from 8.7 to 10.0 was tested.Figs. 1a and b exhibited that running buffer pH indeed had significant effect on the OT-CEC separation efficiency. We observed that the peaks of cephalexin and amoxicillin were overlapped (Fig. S7a in Supporting information) at pH 8.7 (Rs2 = 0), while we could obtain the baseline separation at pH 9.0 (Rs1 = 1.6, Rs2 = 2.1) as displayed in Fig. 2c. When the running buffer pH was increased from 9.0 to 10.0, the separation efficiency between cephradine and cephalexin decreased obviously (Fig. S7b–d in Supporting information). It should be noted when pH at higher than 9.0, the migration time of amoxicillinwas prolonged greatly, which might be caused by the dissociation of amoxicillin (pKa = 9.6). Finally, buffer solution pH at 9.0 was selected for further study.
|
Download:
|
| Fig. 1. Effect of pH (a, b), buffer concentration (c, d) and temperature (e, f) on OT-CEC separation efficiency of cephradine (280.0 μmol/L), cephalexin (280.0 μmol/L), amoxicillin (550.0 μmol/L). Buffer conditions: 40.0mmol/L borax, pH 8.7–10.0, 25 ℃ (a, b); 20.0–60.0 mmol/L borax, pH 9.0, 25 ℃ (c, d); 40.0mmol/L borax, pH 9.0. 25 ℃ and 45 ℃ (e, f). OT-CEC conditions were same as Fig. S7. Rs1 was Rs between cephradine and cephalexin; Rs2 was Rs between cephalexin and amoxicillin; t1, t2, t3 was the migration time of cephradine, cephalexin and amoxicillin, respectively. | |

|
Download:
|
| Fig. 2. Electrophoretograms of the three test β-lactam antibiotics. Using (a) bare capillary with 40.0mmol/L borax as buffer solution (pH 9.0); (b) bare capillary with 40.0mmol/L borax and 140.0mmol/L SDS as buffer solution (pH 9.0); (c) coated capillary with 40.0mmol/L borax as buffer solution at pH 9.0. Other conditions were same as in Fig. 1. Peaks: 1. Cephradine (280.0 μmol/L); 2. cephalexin (280.0 μmol/L); 3. amoxicillin (550.0 μmol/L). | |
Figs. 1c and d describe that the running buffer concentrations ranging from 20.0 mmol/L to 60.0 mmol/L displayed some influence on the OT-CEC separation efficiency of the test analytes. The migration time of the test analytes was prolonged with the buffer concentration increasing, while the three test analytes could not be baseline separated when the concentration of the buffer solution was 20.0 mmol/L or 30.0 mmol/L (Figs. S8a and b in Supporting information). The baseline separation of the test analytes could be obtained with 40.0 mmol/L borax as the buffer solution (Fig. 2c). While, the migration time of the test drugs was prolonged with 50.0 mmol/L borax or 60.0 mmol/L borax (Figs. S8c and d in Supporting information). The concentration of the running buffer affects the ionic strength, by which, a higher concentration of the running buffer induced a lower value of electroosmotic flow (EOF), and prolonged the migration time of the analytes [15]. Moreover, the influence of different buffer solutions on separation efficiency was investigated. But the three analytes could not be baseline separated with phosphate or ammonium acetate buffer. In order to get the highest Rs within a relative short migration time, 40.0 mmol/L borax buffer solution was chosen for further research.
Next, the species and the amount of organic additives in running buffer were investigated. As showed in Table S1 in Supporting information, we found that although Rs1 could increase when 10% methanol was added into the running buffer, the migration time of cephradine and cephalexin was prolonged. Moreover, although Rs2 could be enhanced by adding 10% acetonitrile, tetrahydrofuran or acetone into the buffer solution (v/v), respectively, cephradine and cephalexin still could not be baseline separated. Thus, no organic additives were added into the buffer solution for further OT-CEC study.
In order to investigate the influence of polymer concentration on the separation efficiency, the coating capillaries with different concentrations of polymer were prepared. Table S2 in Supporting information showed that once the polymer concentrations were increased from 10.0mg/mL to 25.0mg/mL, the separation efficiency of the selected analytes was significantly improved, and then decreased when the polymer concentrations was further increased to 45.0mg/mL. This might be caused by the increased viscosity of high polymer concentrations, which made the coating process more difficult. Finally, 25.0mg/mL was selected for separation of the selected β-lactam antibiotics. Further, effect of polymer coating thickness through different coating time on the separation efficiency was studied. Fig. S9b in Supporting information depicts that longer coating time (3.0h) resulted in prolonged migration time. While, shorter coating time (0.5h) induced part separation of the test analytes (Fig. S9a). Obviously, 1.5h coating time could realize the best separation result (Fig. 2c).
It is well known that PNIPAm is a kind of thermo-responsive polymer with the lower critical solution temperature (LCST) around at 32 ℃ [16]. Effect of thermo-responsive property of the resultant P(MAn-St-NIPAm) on the separation efficiency was further investigated because the changed temperature in OT-CEC process would change the hydrophobic/hydrophilic properties of the coating [17]. As exhibited inFigs.1e and f, we observed that comparing with the results obtained at 25 ℃ (< LCST), when the OT-CEC was processed at 45 ℃ (>LCST), Rs1 was slightly changed, Rs2 was increased greatly with the prolonged migration time (Fig. S10 in Supporting information). It could be explained that at higher temperature, the property of PNIPAm moiety in the block copolymer would change from hydrophilicity to hydrophobicity, which was propitious to get better separation efficiency and led to prolonger migration time. Meanwhile, higher temperature would negatively influence the heat transfer in the electrophoresis process. Therefore, OT-CEC at 25 ℃ (Fig. 2c) was selected for further study. Notably, the thermo-responsive property of the preparedblockco-polymercould provide a newidea for separation and analysis of neutral analytes.
Fig. 2a clearly reveals that the three test analytes could not be well separated by capillary zone electrophoresis with bare capillary.After 140.0mmol/L SDS was added into the running buffer, the migration order of the test three drugs was changed (Fig. 2b).Itshouldbenoted that although the separation efficiency of MEKC was dramatically enhanced, the selected three analytes still could not be completely separated (Fig. 2b, Rs1 < 1.5). While when the prepared P(MAn-StNIPAm) was used as the OT-CEC coating, the test three β-lactam antibiotics could reach the baseline separation (Fig. 2c), indicating a great potential of block co-polymer coatings for improvement the OT-CEC separation efficiency of neutral drugs.
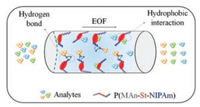
Our results indicated that the unique block co-polymer could be used for well separation of β-lactam antibiotics by OT-CEC. The separation mechanism is described in Fig. 3. The maleic anhydride moiety of P(MAn-St-NIPAm) could chemically bond with the APTES modified capillary, which made the coating more stable; the benzene ring in styrene part could provide the strong hydrophobic interaction with the analytes; PNIPAm moiety of the co-polymer could form hydrogen bonds with -COOH or -OH groups of the analytes at lower temperature (< LCST) [16]. As displayed in Fig. S1, there are both of -OH and -COOH groups in amoxicillin, which would be assumed to form stronger hydrogen bonds with PNIPAm moiety of P(MAn-St-NIPAm), while the other two test analytes only have -COOH groups. Moreover, the hydrophobic interaction between cephradine (LogP = -1.05) [18] or cephalexin (LogP = 0.65) [19] and the PSt part of the block copolymer should be different (Fig. S1). Therefore, the migration order in OT-CEC separation is cephradine > cephalexin > amoxicillin, as depicted in Fig. 2c.
|
Download:
|
| Fig. 3. Mechanism illustration of OT-CEC separation in coated capillary. | |
The stability of coating capillary was investigated by measuring the EOF of thiourea with continuous sampling. Fig. S11 indicates that the coating capillary could withstand continuous sampling for 30 times, and the relative standard deviation (R.S.D) of EOF was 2.2%, which indicated a good stability of coating capillary.
Repeatability between different coating capillaries was further investigated, which was shown in Table S3 in Supporting information.The R.S.D of the migration times of analytes using three different coating capillaries were between 0.43% and 0.61%, while the R.S.D of peak areas were between 0.47% and 0.89%. The results depicted that the coating capillaries had a good repeatability.
The linearity curves of the selected three β-lactam antibiotics obtained in the OT-CEC assay were displayed in Table S4 in Supporting information. The recoveries which were determined by adding known concentration standards into the serum samples were between 95.4%–106.2%, as shown in Table S5 in Supporting information. The results indicated the developed OT-CEC assay with the coating capillary could be applied for determination of the β-lactam antibiotics in serum samples.
The serum samples spiked with the standard solutions were measured by the proposed OT-CEC system, and the results were displayed in Fig. S12 in Supporting information. Fig. S12a is the blank serum sample, which exhibited that there was no interference for determination of β-lactam antibiotics. Fig. S12b shows the electrophoretogram of the serum sample spiked with the three test β-lactam antibiotics, which demonstrates the analytes could be well separated in the real bio-sample by the constructed OT-CEC method.
In summary, we have successfully synthesized a unique block co-polymer P(MAn-St-NIPAm) by RAFT reaction. Subsequently, a new OT-CEC system has been constructed with the prepared block co-polymer as the coating for the first time and applied in analysis of β-lactam antibiotics in serum samples. Owing to the tunable hydrophobic/hydrophilic properties of the block co-polymer, the migration time of the analytes could be controllable. Importantly, comparing with the bare capillary, the OT-CEC separation efficiency of β-lactam antibiotics has been significantly improved without adding surfactants in the buffer solution. Moreover, the coating capillary has displayed its good repeatability and reproducibility. Our protocol has demonstrated a great potential of OT-CEC in providing a new way for synthesis of more novel block co-polymers and for analysis of neutral drugs in real bio-samples without surfactants.
AcknowledgmentsThe authors are grateful for the financial support from the National Natural Science Foundation of China (Nos. 21727809, 21635008, 21621062) and Chinese Academy of Sciences (No. QYZDJ-SSW-SLH034). We also gratefully thank Prof. Ke Zhang for his kind help in GPC measurement.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.03.007.
| [1] |
W.M.A. Niessen, J. Chromatogr. A 812 (1998) 53-75. DOI:10.1016/S0021-9673(98)00281-7 |
| [2] |
V.M. Johnson, J.P. Allanson, R.C. Causon, J. Chromatogr. B 740 (2000) 71-80. DOI:10.1016/S0378-4347(00)00023-2 |
| [3] |
B. Soledad-Rodríguez, P. Fernández-Hernando, R.M. Garcinuño-Martínez, et al., Food. Chem. 224 (2017) 432-438. DOI:10.1016/j.foodchem.2016.11.097 |
| [4] |
M. Carlier, V. Stove, J.J. De Waele, et al., J. Chromatogr. B 978-979 (2015) 89-94. DOI:10.1016/j.jchromb.2014.11.034 |
| [5] |
C.S. Wu, J.L. Zhang, Y. Ling, et al., Chin. Chem. Lett. 22 (2011) 334-337. DOI:10.1016/j.cclet.2010.09.035 |
| [6] |
Y. Jiang, M.Y. He, W.J. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1640-1652. DOI:10.1016/j.cclet.2017.05.008 |
| [7] |
W. Jin, W.Y. Wang, Y.L. Zhang, et al., Chin. Chem. Lett. 24 (2013) 636-638. DOI:10.1016/j.cclet.2013.04.023 |
| [8] |
M. Castro-Puyana, A.L. Crego, M.L. Marina, Electrophoresis 31 (2010) 229-250. DOI:10.1002/elps.v31:1 |
| [9] |
K. Michalskaa, G. Pajchel, S. Tyski, J. Chromatogr. A 1216 (2009) 2934-2942. DOI:10.1016/j.chroma.2008.08.025 |
| [10] |
M. Molina, M. Silva, Electrophoresis 23 (2002) 3907-3921. DOI:10.1002/elps.200290009 |
| [11] |
S. Akamatsu, T. Mitsuhashi, J. Sep. Sci. 37 (2014) 304-307. DOI:10.1002/jssc.v37.3 |
| [12] |
Van Biesen G., C.S. Bottaro, Electrophoresis 27 (2006) 4456-4468. |
| [13] |
Y. Shen, L. Qi, J.L. Qin, et al., Talanta 84 (2011) 501-507. DOI:10.1016/j.talanta.2011.01.039 |
| [14] |
C. Zhang, L.J. Chen, L. Tan, et al., React. Funct. Polym. 93 (2015) 190-201. DOI:10.1016/j.reactfunctpolym.2015.05.009 |
| [15] |
S. Zhang, S.Q. Dong, L.Z. Chi, et al., Talanta 76 (2008) 780-784. DOI:10.1016/j.talanta.2008.04.025 |
| [16] |
C. Wu, Polymer 39 (1998) 4609-4619. DOI:10.1016/S0032-3861(97)10130-6 |
| [17] |
X. Zhang, L.A. Colón, Electrophoresis 27 (2006) 1060-1068. |
| [18] |
V. Karalis, A.T. Kakoulidou, P. Macheras, Eur. J. Pharm. Sci. 20 (2003) 115-123. DOI:10.1016/S0928-0987(03)00177-5 |
| [19] |
P.A. Gediya, D.D.J. Sen, Int. J. Adv. Pharm. Res. 4 (2013) 2071-2076. |
 2019, Vol. 30
2019, Vol. 30 

