b Shanghai Collaborative Innovation Center for Biomanufacturing Technology, Shanghai 200237, China
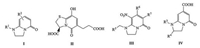
Bicyclic pyridones are a mainstay in chemistry of medicine, agrochemicals and fluorescent development, which are found in biologically active natural products [1] {Perreault, 2010 #2} {Perreault, 2010 #2}. As reported, A series of substituted bicyclic pyridones have become a basis for anti-inflammatory [2, 3] (Fig. 1, Ⅰ), anti-cancer agents [4] and acetylcholinesterase inhibitors [5] (Fig. 1, Ⅱ). For example, imidacloprid derivatives containing bicyclic pyridone fragment were reported [6] (Fig. 1, Ⅲ) as insecticide by Bayer. Besides, bicyclic pyridone have recently been considered as fluorescent derivatives [7, 8], and also serve as the main building blocks to self-assemble into supramolecular nanodots [9] (Fig. 1, Ⅳ).
|
Download:
|
| Fig. 1. Active compounds that possess bicyclic pyridone framework. | |
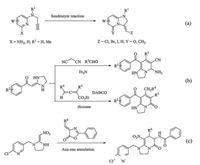
The reported synthesis methods of bicyclic pyridones Ⅰ are noteworthy and mainly based on follow approaches: (1) the cyclocondensation of ketenaminals [10-12], (2) the addition of diamines to cyanobutenoic esters [13], and (3) the addition of diamines to halo- or thiomethylpyridones [14]. Moreover, the survey of literatures reveals a few early examples on the synthesis of bicyclic pyridines. David team developed a practical two-step procedure in which N-(prop-2-yn-1-yl)pyridin-2-amines undergoes Sandmeyer reaction [15] (Scheme 1a). Besides, other two approaches which used polarized N, N-acetals to synthesize bicyclic pyridones [11, 12] were described in Scheme 1b. Our previous work reported that the synthesis of tetrahydroimidazo [1,2-a]pyridin-5(1H)-one derivatives were synthesized through Aza-ene annulation [16] (Scheme 1c). Recent methods included halocyclization of allylamino pyridines [17], intramolecular Mitsunobu reaction, hydrolysis of a bicyclic pyridinium salt intermediate [18] and cyclic condensation of heterocyclic ketene aminals with b-keto ester enol tosylates [19, 20].
|
Download:
|
| Scheme 1. Approaches to synthesis of imidazole pyridone derivatives. | |
Considering the importance of imidazole pyridones groups in pharmaceuticals and agrochemicals, it was necessary to develop a novel and simple methodology. 2-(1-((6-Chloropyridin-3-yl)methyl)imidazolidin-2-ylidene)-2-nitroacetaldehyde (1) is a high active and common intermediate in many fields. Under the motivation, we synthesized (Z)-2-(1-((6-chloropyridin-3-yl)methyl)imidazolidin-2-ylidene)-2-nitroacetaldehyde (2) using 1 as the starting material and developed a new synthesis method of imidazole pyridone 4 starting from 2 and 2-aminoacetic acid methyl ester hydrochloride and characterized it well by spectroscopic techniques. Amide structure is common and focused scaffold in various pesticides [21-24] and natural product [25]. In order to increase the biological activity, the generated bicyclic pyridone intermediate (4) was considered to be amidated further.
The general synthesis procedures are shown in Scheme 2. Our route to synthesize the target compounds began with 1 and DMFDMA which underwent condensation to provide 1'. Hydrolysis of 1' gave 2 as the substrate of the key intermediate 4 and then amidation derivatives were obtained. The details are given in the Supporting information.
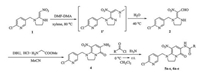
|
Download:
|
| Scheme 2. General procedure for the synthesis of 5a-s, 6a-e. | |
At first, the reaction of key intermediate 4 was optimized. Compound 2 was selected as the model substrate to optimize the reaction conditions. The solubility of 2-aminoacetic acid methyl ester hydrochloride A in MeCN is moderate, while the replacement of MeCN by other solvents brought inferior yields (the data were not shown). Therefore, a variety of bases were explored using MeCN as the solvent (Table 1, entries 1–17). Initially, the screening of the bases revealed that base played a unique role in this transformation. As shown in Table 1, the conversion rates of substrates were over 90% by the treatment of 3.0 equiv. of organic bases with 2.5 equiv. of A in refluxing MeCN (entries 1–12), while inorganic bases showed lower catalytic efficiency (entries 13–17) which could probably be explained by the poor solubility in MeCN. It can be observed that product 3 was obtained easily in good yields and high selectivity with Et3N or DIPEA as base (entries 3 and 4), whereas low yields and selectivity for 4. Among them, 20.7% yield with DBU was better and selected for further optimization, which was investigated under a different equivalent/time/temperature combination (entries 18–27). The ratio of A and DBU was investigated first, and the conversion of 4 was better when 4.0 equiv. A and 2.5 equiv. DBU were selected (entries 18–22). Basing on this results, we conjectured that the instability of 2 resulted high conversion rate together with low yield in the relatively fastidious reflux conditions. Keeping this in mind, we hypothesized that it was important to choose mild room temperature while extended the reaction time to improve the selectivity (entries 23–27). After the optimization, 4 could be given with high selectivity despite of the decreased conversion rate. In summary, when compound 2 was treated with A (4.0 equiv.) and DBU (2.5 equiv.) (entry 23) in MeCN at room temperature for 96 h, the reaction proceeded effectively, affording 4 in 52%.
|
|
Table 1 Optimization for reaction conditions of intermediate 4. |
Target products 5a-s, 6a-e were synthesized from compound 4 and acyl chloride (Scheme 2). For substituted phenyl amide, different substituents were introduced into phenyl ring including halogen, methyl, methoxyl, cyano, trifluoromethoxyl and so on. The spectrum data of representative compound 4 and 6a were given as follows.
6-Amino-1-((6-chloropyridin-3-yl)methyl)-8-nitro-2, 3-dihydroimidazo[1,2-a]pyridin-5(1H)-one (4). Red powder, yield 49.3%; IR (KBr, cm-1): 3436, 3337, 1671, 1630, 1574, 1449, 1396, 1332, 1274, 1236, 1203; Mp 181.9–182.4 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.40 (d, 1H, J = 2.4 Hz), 7.86 (dd, 1H, J1 = 2.4 Hz, J2 = 8.4 Hz), 7.52 (d, 1H, J = 8.4 Hz), 7.03 (s, 1 H), 4.91 (s, 2H), 4.70 (s, 2H), 4.13–3.83 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 157.0, 149.6, 149.4, 146.6, 139.4, 132.6, 129.9, 124.5, 114.7, 106.7, 52.6, 51.2, 43.7; HRMS (ESI+) calcd. for C13H12ClN5O3 (M + Na)+: 344.0526; Found: 344.0521.
N-(1-((6-Chloropyridin-3-yl)methyl)-8-nitro-5-oxo-1, 2, 3, 5- tetrahydroimidazo[1,2-a]pyridin-6-yl)acetamide (6a). Yellow powder, yield 93.0%; IR (KBr, cm-1): 3481, 1651, 1619, 1526, 1466, 1400, 1320, 1284; Mp 220.7–222.0 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.97 (s, 1H), 8.38 (s, 1H), 7.81-7.77 (m, 2H), 7.37 (d, 1H, J = 8.0 Hz), 4.74 (s, 2H), 4.27–3.82 (m, 4H); 13C NMR (100 MHz, DMSO-d6): δ 168.5, 156.1, 151.7, 149.0, 146.6, 138.7, 129.7, 124.8, 120.5, 119.3, 115.0, 52.5, 50.2, 43.1, 24.5; HRMS (EI+) calcd. for C15H1435ClN5O4 (M)+: 363.0734; Found: 363.0730; Calcd. for C15H1437ClN5O4 (M)+: 365.0705; Found: 365.0729.
Bioassays were performed on representative test organisms grown in the laboratory. The insecticidal activities of the synthetic compounds against Tetranychus cinnabarinus, Aphis craccivora, Prodenia litura and Nilaparvata lugens were tested according to our previous reported procedure [26-28], and detailed procedures are given in the Supporting information.
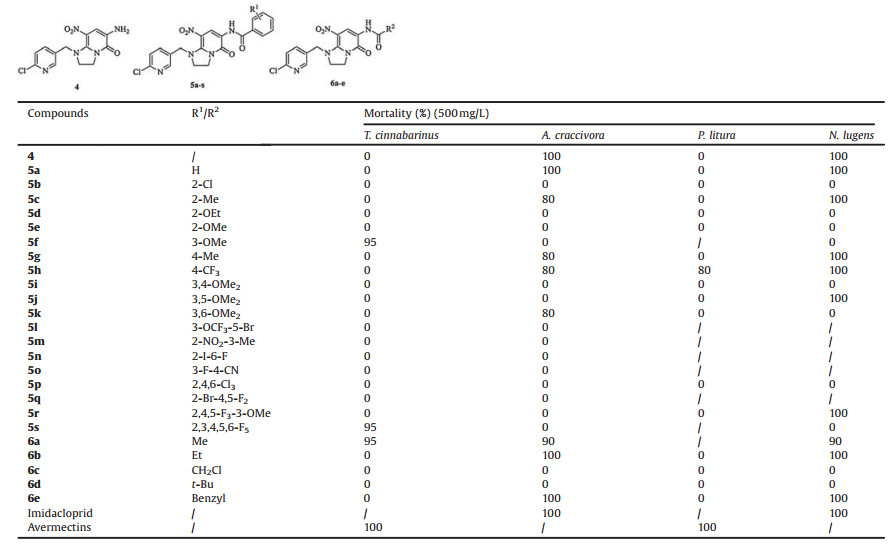
The results of bioassay are depicted in Table 2, precursor compound 4 showed 100% mortality against A. craccivora and N. lugens at 500 mg/L. Compounds 5a-s, 6a-e were the derivatives of compound 4. No substituent (5a), moderate electron-donating and electron-withdrawing (5c, 5g, 5h) had good activity against A. craccivora and N. lugens. However, electron-withdrawing Cl group (5b) and strong electron-donating (5d, 5e), showed low activity. Meanwhile, when introducing disubstituted and polysubstituted groups into benzene ring except 5i, 5l, 5m, 5n, 5o, 5p, 5q, the other compounds exhibited the insecticidal activity. For aliphatic amide, derived alkyl groups (6a, 6b, 6e) exhibited 90%–100% mortality against A. craccivora and N. lugens. Compounds 5f, 5s and 6a showed 95% mortality against T. cinnabarinus. Among them, only 6a still kept the activity against A. craccivora and N. lugens, besides T. cinnabarinus.
|
|
Table 2 Insecticidal activity test result of targeted compounds (500 mg/L). |
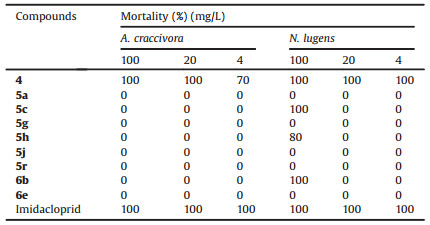
Furthermore, the compounds with 100% mortality at 500 mg/L compounds 4, 5a, 5c, 5g, 5h, 5j, 5r, 6b, 6e were selected to investigate their activity at lower concentration. As shown in Table 3, the result was undesirable, most compounds almost completely lost the activity except 5c and 6b which exhibited 100% mortality against N. lugens at 100 mg/L. But when the concentration further decreased, the bioactivity of 5c and 6b sharply decreased and disappeared completely. To our surprise, precursor compound 4 kept its activity at 4 mg/L and the bioactivity still reached 70% and 100% mortality against A. craccivora and N. lugens, respectively. It meant that it could be used as a lead for further derivation.
|
|
Table 3 Insecticidal activity of some targeted compounds against A. craccivora and N. lugens. |
In summary, we have developed an efficient synthetic approach of bicyclic pyridone 4 starting from 2 and 2-aminoacetic acid methyl ester hydrochloride, which can be used to synthesize various bicyclic pyridones by the replacement of chloropyridine ring. And then a series of amidation derivatives of 4 were synthesized. Their structures were characterized by 1H NMR, 13C NMR, 19F NMR and HRMS. The bioassays showed that some of the target compounds exhibited excellent insecticidal activities against brown planthopper (N. lugens) (4, 5a, 6b, 6e), cowpea aphids (A. craccivora) (4, 5a, 5c, 5g, 5h, 5j, 5r, 6b, 6e) and T. cinnabarinus (5f, 5s, 6a) at 500 mg/L. Despite the activities of most derivatives were undesirable at low concentration, precursor compound 4 kept high activity and broad insecticidal spectra on N. lugens and A. craccivora and could be used as the lead for further derivation. The further structural optimizations are in progress.
AcknowledgmentsThis work was financial supported by the National Key Research Program of China (No. 2017YFD0200500), National Natural Science Foundation of China (No. 21672061). This work was also supported by the Fundamental Research Funds for the Central Universities (No. 222201718004).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.09.012.
| [1] |
S. Perreault, T. Rovis, Chem. Soc. Rev. 41 (2010) 3149-3159. |
| [2] |
K. Kubo, N. Ito, I. Souzu, et al., Patent, US4186200, 1980.
|
| [3] |
Y.M. Lee, F. Almqvist, S.J. Hultgren, Curr. Opin. Pharm. 3 (2003) 513-519. DOI:10.1016/j.coph.2003.04.001 |
| [4] |
M.E. Wall, M.C. Wani, C.E. Cook, et al., J. Am. Chem. Soc. 88 (1966) 3888-3890. DOI:10.1021/ja00968a057 |
| [5] |
B.S. Wang, H. Wang, Z.H. Wei, et al., J. Neural Transm. 116 (2009) 457-465. DOI:10.1007/s00702-009-0189-x |
| [6] |
K. Shiokawa, S. Tsuboi, S. Sasaki, et al., Patent, US5472960, 1995.
|
| [7] |
J. Schneider, C.J. Reckmeier, Y. Xiong, et al., J. Phys. Chem. 121 (2017) 2014-2022. DOI:10.1021/acs.jpca.6b11814 |
| [8] |
Y. Song, S. Zhu, S. Zhang, et al., J. Mater. Chem. C Mater. Opt. Electron. Devices 3 (2015) 5976-5984. DOI:10.1039/C5TC00813A |
| [9] |
X. Meng, Y. Wang, X. Liu, et al., Opt. Mater. 77 (2018) 48-54. DOI:10.1016/j.optmat.2018.01.005 |
| [10] |
M.X. Zhao, M.X. Wang, Z.T. Huang, Tetrahedron 58 (2002) 1309-1316. DOI:10.1016/S0040-4020(02)00002-9 |
| [11] |
L.R. Wen, C.Y. Jiang, M. Li, L.J. Wang, Tetrahedron 67 (2011) 293-302. DOI:10.1016/j.tet.2010.11.049 |
| [12] |
M. Li, Z.M. Zhou, L.R. Wen, Z.X. Qiu, J. Org. Chem. 76 (2011) 3054-3063. DOI:10.1021/jo102167g |
| [13] |
J.L. Buchanan, D. Elbaum, M.W. Martin, et al., Patent (2008) US7442698.
|
| [14] |
D.G. Hehemann, W. Winnik, J. Heterocycl. Chem. 31 (1994) 393-396. DOI:10.1002/jhet.v31:2 |
| [15] |
D. Sucunza, A. Samadi, M. Chioua, et al., Chem. Commun. 42 (2011) 5043-5045. |
| [16] |
X.Q. Liu, Y.Q. Liu, X.S. Shao, et al., Chin. Chem. Lett. 27 (2016) 7-10. DOI:10.1016/j.cclet.2015.10.002 |
| [17] |
S. Schmid, D. Schühle, S. Steinberger, Z. Xin, V. Austel, Synthesis 2005 (2005) 3107-3118. DOI:10.1055/s-2005-918409 |
| [18] |
D. Cheng, L. Croft, M. Abdi, A. Lightfoot, T. Gallagher, Org. Lett. 9 (2007) 5175-5178. DOI:10.1021/ol701689z |
| [19] |
S.J. Yan, Y.F. Niu, R. Huang, J. Lin, Synlett 2009 (2009) 2821-2824. DOI:10.1055/s-0029-1217984 |
| [20] |
L. Hu, K.M. Wang, M. Zhao, et al., Tetrahedron 70 (2014) 4478-4484. DOI:10.1016/j.tet.2014.05.032 |
| [21] |
B.L. Wang, H.W. Zhu, Y. Ma, et al., J. Agric. Food Chem. 61 (2013) 5483-5493. DOI:10.1021/jf4012467 |
| [22] |
S. Zhou, S. Zhou, Y. Xie, et al., Chin. Chem. Lett. 29 (2018) 1254-1256. DOI:10.1016/j.cclet.2017.10.022 |
| [23] |
J.J. Shi, G.H. Ren, N.J. Wu, et al., Chin. Chem. Lett. 28 (2017) 1727-1730. DOI:10.1016/j.cclet.2017.05.015 |
| [24] |
L.Y. Zhang, B.L. Wang, Y.Z. Zhan, et al., Chin. Chem. Lett. 27 (2016) 163-167. DOI:10.1016/j.cclet.2015.09.015 |
| [25] |
R. Yang, Y. Guo, Y. Zhang, H. Xu, Chin. Chem. Lett. 29 (2018) 995-997. DOI:10.1016/j.cclet.2017.10.018 |
| [26] |
Z. Tian, X. Shao, Z. Li, X. Qian, Q. Huang, J. Agric. Food Chem. 55 (2007) 2288-2292. DOI:10.1021/jf063418a |
| [27] |
S. Lu, X. Shao, Z. Li, et al., J. Agric. Food Chem. 60 (2012) 322-330. DOI:10.1021/jf203068a |
| [28] |
X. Shao, H. Fu, X. Xu, et al., J. Agric. Food Chem. 58 (2010) 2696-2702. DOI:10.1021/jf902531y |
 2019, Vol. 30
2019, Vol. 30