b Key Laboratory of Synthetic Rubber, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China
Molybdenum disulfide (MoS2), a graphene-like two-dimensional (2D) layered material, has recently exhibited expanding potentials in diverse applications, e.g., optoelectronics [1-4], catalysis [5, 6], sensing [7, 8], and biomedical fields [9, 10], due to its unique optical, electronic and catalytic properties. A variety of approaches including chemical vapor deposition growth [11, 12], chemical exfoliation [13, 14], or direct exfoliation in solvents [15], can lead to the production of ultra-thin and high-quality 2D MoS2. To meet the requirement for diverse applications, the functionalization of MoS2 is highly demanded to enhance its processibility, improve the compatibilities with solvents/environments and further modulate the electronic properties. However, due to its relatively inert character, functionalization of MoS2 is usually more difficult than its graphene counterpart, e.g., functionalities can be easily introduced onto graphene after oxidization [16]. Most covalent functionalization strategies rely on sulfur chemistry in which thiol ligands containing functional groups or polymers were chemically conjugated to S vacancy defect on MoS2 nanosheets [17, 18]. With the rapid development of MoS2-based nanomaterials and increasing demand for diverse applications, more facile and efficient methods in tailoring 2D MoS2 with synthetic polymers is demanded.
The covalent modification of surfaces with functional polymer brushes has received increasing interest due to potential applications in sensing [19], combinatorial science [20], medicine [21], and anti-icing technology [22]. Polymer brushes provide the possibility in tailoring the surface functionalities with multiple functional sites on each grafted polymer chain while allowing for the fine-tuning of the topological architectures with the aid of living/controlled polymerization techniques [23]. Furthermore, covalently bound polymer chains provide both stable and soft environments which are compatible with further functionalization [24, 25]. Surface-initiated polymerization from chemically defined self-assembled monolayers on solid surfaces has been widely utilized for the formation of polymer brush layers with well controlled functionality, thickness, and density with high precision [26].
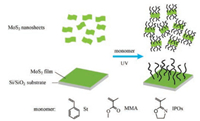
Self-initiating photografting and photopolymerization (SIPGP) is a straightforward "grafting from" strategy which can covalently create grafted polymers on surface without the immobilization of initiators on the surface [27, 28]. As a facile surface modification method, the grafting reaction can be performed at room temperature and mild reaction conditions, avoiding the employment of chemical reactions in the presence of harsh conditions and elevated temperature. Thus, a variety of surfaces such as graphene, silicon, silicon carbide, glassy carbon, diamond, vanadium carbides, TiO2 substrates, etc. could be modified via the abstraction of H from surfaces with relatively low bond dissociation energy of H-substrate [29-36]. Herein, we demonstrate it is possible to tailor the MoS2 surface with different type of polymer brushes ranging from traditional PMMA, polystyrene to functional PIPOx brushes. We show that the selective modification, i.e., the formation of polymer brushes on one side or both sides of MoS2 could be achieved by performing the SIPGP using MoS2 suspension or deposited nanosheets on flat substrates.
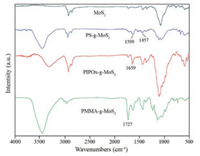
As outlined in Scheme 1, self-initiated photografting and photopolymerization (SIPGP) of various monomers, i.e., methyl methacrylate (MMA), styrene (St) and 2-isopropenyl-2-oxazoline (IPOx) was firstly attempted on exfoliated MoS2 nanosheets via UV irradiation with a spectral distribution between 250 nm and 350 nm (λmax= 300 nm). After the grafting polymerization, the modified MoS2 nanosheets were rigorously ultrasonicated in different solvents with different polarities and subsequently centrifuged to remove physisorbed polymers and unreacted monomers. Infrared (IR) spectroscopy was used to confirm the formation of polymer brushes on MoS2 nanosheets. As shown in Fig. 1, peaks at 1727 cm-1 attributed to the stretching vibration of C=O and 1147 cm-1 originates from the C—O stretching mode indicating the successful formation of poly(methyl methacrylate) PMMA grafts on MoS2. Strong band at 1659 cm-1 is attributed to the stretching vibration of C=N from oxazoline rings, indicating the successful formation of poly(2-isopropenyl-2-oxazoline) (PIPOx) brushes on the nanosheets. The appearance of peaks between 1599 cm-1 and 1457 cm-1 corresponding to the stretching of C=C of benzene rings, demonstrating the existence of polystyrene (PS) grafts on MoS2 nanosheets. Therefore, the UVinduced photografting polymerization allows the direct formation of a series of polymer brushes on the MoS2 nanosheets without any immobilization of initiators on the MoS2 surface.
|
Download:
|
| Scheme 1. Preparation of polymer grafts on MoS2 nanosheets and monolayer deposited on a silicon wafer substrate. | |
|
Download:
|
| Fig. 1. IR spectra for exfoliated MoS2 and polymer grafts of PS, PIPOx and PMMA brushes on MoS2 nanosheets. | |
It was demonstrated that the exfoliation process may produce structural defects such as S vacancy defect in the MoS2 surface. The formation of polymer brushes on the MoS2 surface is probably because of the grafting polymerization on these defects. To further confirm the formation of polymer brushes and gain insight into the grafting reaction, the PMMA modified MoS2 nanosheets were characterized by X-ray photoelectron spectroscopy (XPS). The peaks at 284.6 and 532.9 eV could be assigned to the C 1s and O 1s from PMMA (Fig. 2). The C 1s peak can be deconvoluted into four components at 284.5, 285.2, 288.6, and 286.0 eV corresponding to the C=C, C—O, O—C=O and C—S, respectively. The XPS measurements corroborate the formation of PMMA brushes on MoS2 and further indicate the generation of C—S bond between PMMA and substrate during the grafting polymerization.

|
Download:
|
| Fig. 2. The C 1s high-resolution XPS spectrum of PMMA grafts onMoS2 nanosheets. | |
In order to investigate the morphology and thickness change, the resulted nanosheets were characterized by AFM. As shown in Fig. S1 in Supporting information, pristine MoS2 has an average thickness less than 1 nm. This indicates the obtained MoS2 are almost single layered. After the photografting reaction, the thickness increased to 21 nm (Fig. S2 in Supporting information), indicating the successful occurrence of the grafting reaction. Since the chemical compositions on both sides of MoS2 nanosheet are identical, thus the grafting reaction should occur on both sides of MoS2 during the grafting polymerization.
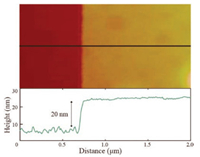
Asymmetric functionalization of two dimensional (2D) nanosheets or membranes is of great interest, which can endow the 2D nanosheets with different properties leading to fascinating functions, e.g., self-assembled behaviors [18, 37-39]. In order to demonstrate the MoS2 surface can be modified asymmetrically, we performed the UV grafting polymerization on MoS2 monolayer deposited on a silicon wafer. Following the above-mentioned procedure, the silicon wafer substrate with a MoS2 monolayer deposited via chemical vapor deposition (CVD) on the top layer was submerged in bulk monomer for the SIPGP. Monolayer of MoS2 is a commercial product, which was prepared by CVD growth technique without obvious defects (Fig. S3 in Supporting information). The interaction between MoS2 and the substrate is van der-waals forces. These forces are strong enough in making the MoS2 layer remain adhered to the substrate during the grafting polymerization. This can be verified by the AFM measurement on the scratched area after the photopolymerization using a sharp needle. The height of the pristine commercial available monolayer MoS2 film on Si/SiO2 measured by AFM is around 0.7 nm. After SIPGP of MMA for 5 h, a homogeneous polymer layer with an average thickness of 20 nm was obtained (Fig. 3). The surface roughness was found to be 0.437 nm (Ra) for PMMA-g-MoS2 nanosheets and 0.112 nm for pristine MoS2 monolayer.
|
Download:
|
| Fig. 3. AFM scan on a scratched area along with a section analysis of the PMMA grafts on MoS2 monolayer on Si/SiO2. | |
During the photografting polymerization, bulk photopolymerization of monomers occurred simultaneously, resulting in the formation of free polymers in solution. According to our previous report, the SIPGP proceeds via a grafting from fashion. Moreover, the degree of polymerization for the grafted polymer chains and polymers formed simultaneously in solution are almost identical [35]. In our case, gel permeation chromatography (GPC) analysis of the PMMA obtained simultaneously from bulk solution gave a high molecular weight and a relatively broad polymer molecular weight distribution (Mn = 4.54 ×105 g/mol, PDI = 1.92, Fig. S4 in Supporting information), which are typical of polymers obtained byfree radical polymerization. Therefore, we estimate the degree of the polymerization of the grafted PMMA is about 4000.
In summary, we have provided a simple and robust strategy in the direct creation of polymer grafts on MoS2 surfaces. A series of polymer class can be anchored on the MoS2 surfaces. The polymer brushes can be created onto one side or both sides of the MoS2 surfaces by performing the grafting polymerization using the MoS2 dispersion or monolayer deposited on the silicon substrate, allowing the selective functionalization of MoS2. The presented approach is of promising potential in applications requiring tailored polymer composition on the MoS2 surfaces. Further investigation in the preparation of free-standing polymer carpets on MoS2 monolayer and their applications is underway.
AcknowledgmentsThe financial support of this work from the Department of Science and Technology of Jilin Province (Nos. 20180101196JC and 20180101170JC) is greatly acknowledged. N. Zhang thanks the support of Youth Innovation Promotion Association CAS (No. 2016207).
Appendix A. Supplementary dataSupplementary materialrelated tothis article canbefound, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2018.07.002.
| [1] |
B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti, A. Kis, Nat. Nanotechnol. 6 (2011) 147-150. DOI:10.1038/nnano.2010.279 |
| [2] |
K.F. Mak, C. Lee, J. Hone, J. Shan, T.F. Heinz, Phys. Rev. Lett 105 (2010) 136805. DOI:10.1103/PhysRevLett.105.136805 |
| [3] |
Z.Y. Yin, H. Li, L. Jiang, et al., ACS Nano 6 (2012) 74-80. DOI:10.1021/nn2024557 |
| [4] |
M.L. Tsai, S.H. Su, J.K. Chang, et al., ACS Nano 8 (2014) 8317-8322. DOI:10.1021/nn502776h |
| [5] |
C.T. Tye, K.J. Smith, Catal. Today 116 (2006) 461-468. DOI:10.1016/j.cattod.2006.06.028 |
| [6] |
B.K. Miremadi, S.R. Morrison, J. Catal. 103 (1987) 334-345. DOI:10.1016/0021-9517(87)90125-4 |
| [7] |
C. Zhu, Z. Zeng, H. Li, et al., J. Am. Chem. Soc. 135 (2013) 5998-6001. DOI:10.1021/ja4019572 |
| [8] |
H. Li, Z.Y. Yin, Q.Y. He, et al., Small 8 (2012) 63-67. DOI:10.1002/smll.201101016 |
| [9] |
W. Yin, L. Yan, J. Yu, et al., ACS Nano 8 (2014) 6922-6933. DOI:10.1021/nn501647j |
| [10] |
S.S. Chou, B. Kaehr, J. Kim, et al., Angew. Chem. 125 (2013) 4254-4258. DOI:10.1002/ange.201209229 |
| [11] |
Y. Zhan, Z. Liu, S. Najmaei, P.M. Ajayan, J. Lou, Small 8 (2012) 966-971. DOI:10.1002/smll.201102654 |
| [12] |
A. Berkdemir, H.R. Gutierrez, Botello-Mendez A.R., et al., Sci. Rep 3 (2013) 1755. DOI:10.1038/srep01755 |
| [13] |
M. Chhowalla, H.S. Shin, G. Eda, et al., Nat. Chem. 5 (2013) 263-275. DOI:10.1038/nchem.1589 |
| [14] |
G. Eda, H. Yamaguchi, D. Voiry, et al., Nano Lett. 11 (2011) 5111-5116. DOI:10.1021/nl201874w |
| [15] |
V. Nicolosi, M. Chhowalla, M.G. Kanatzidis, et al., Science 340 (2013) 1226419. DOI:10.1126/science.1226419 |
| [16] |
C. Backes, N.C. Berner, X. Chen, et al., Angew. Chem. Int. Ed. 54 (2015) 2638-2642. DOI:10.1002/anie.201409412 |
| [17] |
S.S. Chou, M. De, J. Kim, et al., J. Am. Chem. Soc. 135 (2013) 4584-4587. DOI:10.1021/ja310929s |
| [18] |
L. Zhou, B. He, Y. Yang, Y. He, RSC Adv. 4 (2014) 32570-32578. DOI:10.1039/C4RA04682J |
| [19] |
R.C. Bailey, J.T. Hupp, Anal. Chem. 75 (2003) 2392-2398. DOI:10.1021/ac026391c |
| [20] |
M.P. Stoykovich, H.B. Cao, K. Yoshimoto, L.E. Ocola, P.F. Nealey, Adv. Mater. 15 (2003) 1180-1184. DOI:10.1002/adma.200305059 |
| [21] |
L. Hou, J. Fang, W. Wang, et al., J. Mater. Chem. B 5 (2017) 3348-3354. DOI:10.1039/C7TB00812K |
| [22] |
Z. He, W.J. Xie, Z. Liu, et al., Sci. Adv 2 (2016) e1600345. DOI:10.1126/sciadv.1600345 |
| [23] |
N. Zhang, S. Salzinger, F. Deubel, R. Jordan, B. Rieger, J. Am. Chem. Soc. 134 (2012) 7333-7336. DOI:10.1021/ja3027423 |
| [24] |
C. Padeste, P. Farquet, C. Potzner, H.H. Solak, J. Biomater. Sci. Polym. Ed. 17 (2006) 1285-1300. DOI:10.1163/156856206778667505 |
| [25] |
M.A.C. Stuart, W.T.S. Huck, J. Genzer, Nat. Mater. 9 (2010) 101-113. DOI:10.1038/nmat2614 |
| [26] |
T. Chen, I. Amin, R. Jordan, Chem. Soc. Rev. 41 (2012) 3280-3296. DOI:10.1039/c2cs15225h |
| [27] |
M. Steenackers, A.M. Gigler, N. Zhang, et al., J. Am. Chem. Soc. 133 (2011) 10490-10498. DOI:10.1021/ja201052q |
| [28] |
M. Steenackers, R. Jordan, A. Küller, M. Grunze, Adv. Mater. 21 (2009) 2921-2925. DOI:10.1002/adma.v21:28 |
| [29] |
M. Steenackers, I.D. Sharp, K. Larsson, et al., Chem. Mater. 22 (2010) 272-278. DOI:10.1021/cm903051j |
| [30] |
N. Zhang, M. Steenackers, R. Luxenhofer, R. Jordan, Macromolecules 42 (2009) 5345-5351. DOI:10.1021/ma900329y |
| [31] |
A.A. Reitinger, N.A. Hutter, A. Donner, et al., Adv. Funct. Mater. 23 (2013) 2979-2986. DOI:10.1002/adfm.v23.23 |
| [32] |
J. Yang, L. Hou, B. Xu, et al., Macromol. Rapid Commun. 35 (2014) 1224-1229. DOI:10.1002/marc.v35.13 |
| [33] |
J. Chen, K. Chen, D. Tong, et al., Chem. Commun. 51 (2015) 314-317. DOI:10.1039/C4CC07220K |
| [34] |
H. Bian, X. Dong, S. Chen, D. Dong, N. Zhang, Chin. Chem. Lett. 29 (2018) 171-174. DOI:10.1016/j.cclet.2017.05.011 |
| [35] |
L. Hou, L. Wang, N. Zhang, Z. Xie, D. Dong, Polym. Chem. 7 (2016) 5828-5834. DOI:10.1039/C6PY01008C |
| [36] |
H. Bian, J. Yang, N. Zhang, et al., Polym. Chem. 7 (2016) 1191-1196. DOI:10.1039/C5PY02013A |
| [37] |
J.N. Coleman, M. Lotya, A. O'Neill, et al., Science 331 (2011) 568-571. DOI:10.1126/science.1194975 |
| [38] |
D. Han, P. Xiao, J. Gu, et al., RSC Adv. 4 (2014) 22759-22762. DOI:10.1039/c4ra02826k |
| [39] |
P. Zhou, Q. Wang, C.L. Zhang, et al., Chin. Chem. Lett. 26 (2015) 657-661. DOI:10.1016/j.cclet.2015.04.006 |
 2019, Vol. 30
2019, Vol. 30 

