b Key Laboratory of Molecular Virology & Immunology, Unit of Human Parasite Molecular and Cell Biology, Institute Pasteur of Shanghai, University of Chinese Academy of Science, Shanghai 200031, China
Malaria, a mosquito-borne disease caused by infection with Plasmodium parasites, is the world's most deadly parasitic infection [1, 2]. Almost half the world's population lives in malaria endemic areas, and an estimated 1.2 billion people are at high risk of contracting the disease [3-5]. According to WHO 2016, malaria caused 429, 000 deaths and there were approximately 212 million clinical cases of infection globally in 2015. Since the control of malaria has been severely compromised in recent years by the widespread resistance to nearly all frontline therapeutics which were used for both prophylaxes and treatments [3, 6-8], hence, there is an urgent need for the development and discovery of new antimalarial drugs, which are structurally distinct from existing drugs and endowed with novel mechanisms of action [9].
Cysteine protease falcipain-2 (FP-2) of P. falciparum is an essential hemoglobinase of erythrocytic P. falciparum trophozoites and provides the amino acids for the growth and proliferation of Plasmodium. Many in vitro and in vivo studies have confirmed that inhibitors of FP-2 could block parasite hemoglobin hydrolysis, halt the development of culture parasites, and are effective against murine malaria [10, 11]. P. falciparum dihydrofolate reductase (PfDHFR) has received considerable attention for the prophylaxes and treatments of P. falciparum infection. PfDHFR is one of the key enzymes in the process of DNA replication, and could catalyze 7, 8- dihydrofolate to transform into tetrahydrofolate [12, 13]. Tetrahydrofolate serves as a necessary cofactor in the important onecarbon transfer reactions in the biosynthetic pathways of pyrimidines, purines, and amino acids [14]. Thus, a more powerful pesticidal effect could be achieved by inhibiting FP-2 and PfDHFR simultaneously. Such dual inhibitors might show a good synergetic effect, and overcome the drug-resistance and be capable of providing "a combination therapy" in a single agent [15].
Previously, we reported the first-generation dual inhibitors against FP-2 and PfDHFR based on the compound 27, which was randomly identified by screening FP-2 inhibitors in our laboratory, and gained compound 2o (28) which exhibited a high enzymatic inhibition and a moderate in vivo antimalarial efficacy [15] (Fig. 1). Based on the SAR of the first-generation dual inhibitors [15], to gain new scaffold dual inhibitors with more potencies, a novel series of dual inhibitors were designed, synthesized by displacing 4- fluorophenyl with the uniform pharmacophores of reported PfDHFR inhibitors, 2, 4-diamino heterocyclic fragments (Table S1 in Supporting information). Considering the feasibility of the synthesis and novelty of scaffold, the sulfonamide was displaced with the secondary amine (green, Fig. 1), and the amide position was interchanged (gray, Fig. 1). Therefore, 2, 4-diaminopyrimidine analogues with substituent (R, Fig. 1) at the terminal amide (7–26) were synthesized to obtain better dual target inhibitors and explore the SAR.

|
Download:
|
| Fig. 1. Chemical modification strategies for the second-generation dual inhibitors. (For interpretation of the references to color in the text, the reader is referred to the web version of this article.) | |
First of all, we synthesized all the 2, 4-diaminopyrimidine analogues (7–26) as described in Scheme 1. 2, 4-Diaminopyrimidine-5-carbonitrile (2) was prepared by reaction of guanidine carbonate (1) with ethoxymethylenemalononitrile in EtONa and EtOH in a 71% yield. Further treatment of 2 with Nickel in MeOH under H2 for 24 h gave rise to 2, 4-diaminopyrimidine-5-carbaldehyde (3) in an 80% yield. Subsequent condensation of commercially available 4-nitrophenethylamine hydrochloride (4) with appropriate acids RCOOH in the presence of HOBt, EDCI and DIPEA in N, N-dimethylacetamide afforded N-(4-nitrophenethyl)amide 5 in good yields. Reduction of 5 in the presence of 10% Pd/C and H2 in MeOH overnight in good yields provided N-(4-aminophenethyl)amide (6). Analogues 7–26 were performed by the reaction of 3 and 6, via the condition using NaCNBH3 in MeOH under reflux overnight in good yields (30%–70%) and were characterized by 1H NMR and HRMS. The purity was over 95%, as determined by HPLC analysis (Table S2 in Supporting information). Supplementary data associated with experiment section, reported PfDHFR inhibitors and HPLC analysis data of compounds 7–26 can be found in Supporting information.

|
Download:
|
| Scheme 1. Synthesis of the 2, 4-diaminopyrimidine analogues (7-26). Reagents and conditions: (a) Ethoxymethylenemalononitrile, EtONa/EtOH, 5 ℃, 8 h; (b) Ni/H2, MeOH, 25 ℃, 24 h; (c) HOBt, EDCI, DIPEA, RCOOH, DMA, 25 ℃, overnight; (d) 10% Pd/C, H2, MeOH, reflux; (e) NaCNBH3, MeOH, reflux, overnight | |
Then the twenty analogues were evaluated for FP-2 enzymatic inhibitory activity and for PfDHFR enzymatic inhibitory activity using the cysteine protease inhibitor (E-64) and pyrimethamine as the reference standard in the assay, respectively. And we assessed the inhibition rate (IR) of all the analogues against FP-2 and PfDHFR at 10 μmol/L firstly. A set of analogues with various substituents (R), including electron-donating substituted phenyl ring (9–13), electron-withdrawing substituted phenyl ring (14–17), hetero-aryl rings (18–22) and alkyl groups (23–26), were evaluated for IR against FP-2 and PfDHFR, respectively. From the results in Table 1, 3 analogues, i.e., 11, 14 and 15, were identified as inhibitors against FP-2 with IR > 40% at 10 μmol/L and 19 analogues, i.e., 7–24, 26, were identified as potent inhibitors against PfDHFR with IR > 70% at 10 μmol/L. Therefore, the IC50 value of them for the enzymatic inhibitory activity was further evaluated.
|
|
Table 1 In vitro inhibitory activities against FP-2 and PfDHFR of analogues 7–26 |
Analysis of the data shown in Table 1 revealed some noteworthy observations from the SAR study of analogues 7–26: (1) The inhibitory activities against FP-2 showed the R substituents which were phenyl rings with electron-donating groups (EDGs) preferred 3-position (9 vs.10 and 11 vs.12) and the R substituents which were phenyl rings with electron-withdrawing groups (EWGs) favored 4- position (14 vs. 15 vs. 16); (2) In the studied sets of the R substituents, the potency against FP-2 substantially increased in the order of EWG-aryl > EDG-aryl > (aryl)alkyl; (3) In the test of inhibitory activities against PfDHFR, the R substituents could be well tolerated and 19 analogues displayed high potencies (IR at 10 μmol/L > 70%); the potency against PfDHFR substantially increased in the order of EDG-aryl > (aryl)alkyl > EWG-aryl.
From the results described above, analogue 14 was identified as a potent inhibitor against both FP-2 and PfDHFR (IC50 = 6.8 μmol/L against FP-2, IC50 = 8.8 μmol/L against PfDHFR). Therefore, analogue 14 and the lead compound 28 were next evaluated in the inhibitory activity against the blood stage of the multi-drugsensitive P. falciparum 3D7 strain and P. falciparum Dd2 strain, which carry a phenotype of resistance to chloroquine [16, 17]. Compound 28 displayed poor potencies against 3D7 strain (IR = 7.9% @ 20 μmol/L) and Dd2 strain (IR = 6.2% @ 20 μmol/L). As shown in Fig. S1 (Supporting information), analogue 14 together with artemisinin (Art) were tested in 3D7 cells. Analogue 14 performed micromolar potencies against 3D7 parasites (IC50 = 2.9 μmol/L). Moreover, as shown in Fig. S2 (Supporting information), 14 displayed micromolar potency against Dd2 (IC50 = 1.1 μmol/L) while pyrimethamine presented less effective inhibition against Dd2 (IC50 >10 μmol/L), which indicated analogue 14 could also inhibit the growth of resistant P. falciparum. Furthermore, we evaluated the inhibitory activities of compound 14 against two clinical isolated strains, Fab9 and GB4. To our delight, as shown in Fig. S3 (Supporting information), 14 also displayed micromolar potencies against both strains (IC50 = 2.6 μmol/L against Fab9 and IC50 = 1.0 μmol/L against GB4).
Moreover, to understand the structural basis for the inhibitory activities of the inhibitors against FP-2 and PfDHFR, the 3D binding models of analogue 14 with FP-2 and PfDHFR were studied by molecular docking (Fig. 2). Fig. 2A shows the predicted binding poses of 14 in the catalytic site of FP-2. The amide moiety of 14 directly interacted with Asn156 via H-bonds, meanwhile, the terminal 2, 4-diaminopyrimidine group and secondary amine interacted with Asp18 and Lys20 via H-bonds. For PfDHFR (Fig. 2B), the catalytic subpocket formed around Asp54 were occupied by the 2, 4-diaminopyrimidine group in 14. The 2, 4- diaminopyrimidine ring formed a complicated hydrogen-bond network with Asp54, Ile14, and Ile164, respectively, which contributed significantly to the higher affinity of 14. Moreover, the amide group formed an H-bond with the key residue Arg122.
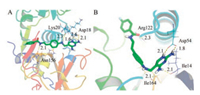
|
Download:
|
| Fig. 2. Molecular docking studies on 14. Docked poses of 14 (green sticks) in the active sites of FP-2 (A) and PfDHFR (B), respectively. Key residues of the binding pockets are shown as lines. Hydrogen bonds are shown with yellow dash lines | |
In summary, we have identified a novel series of 2, 4- diaminopyrimidine analogues (7–26) derived from 28 as FP-2 and PfDHFR dual inhibitors. On the basis of the structure of the lead compound 28, 20 novel 2, 4-diaminopyrimidine analogues have been synthesized and tested in FP-2 and PfDHFR enzymatic inhibitory activity assays. Molecular docking studies showed that the amide and secondary amine groups of new analogues could form hydrogen bonds with the surrounding amino acid residues, which were the same as the first generation dual inhibitors [15], combining with the preliminary SARs, we speculated the amide and secondary amine groups inherited FP-2 inhibitory activity from 28 and 2, 4-diaminopyrimidine groups contributed to the inhibitory activity against PfDHFR. The in vitro inhibitory activity against P. falciparum assays with multi-drug-sensitive strain 3D7 further confirmed that analogue 14 was a good parasites inhibitor (IC50 = 2.9 μmol/L). To our delight, the inhibition of 14 against chloroquine-resistant Plasmodium falciparum strain Dd2 (IC50 = 1.1 μmol/L) was more superior than pyrimethamine (IC50 >10 μmol/L) and analogue 14 displayed micromolar inhibitory activities against two clinical isolated strains Fab9 (IC50 = 2.6 μmol/L) and GB4 (IC50 = 1.0 μmol/L). Overall, 14 has the potential to be developed as a lead compound of antimalarial drugs.
AcknowledgmentsFinancial support for this research provided by the National Natural Science Foundation of China (Nos. 21372001 and 21672064), the "Shu Guang" Project supported by the Shanghai Municipal Education Commission and Shanghai Education Development Foundation (No. 14SG28), and the Fundamental Research Funds for the Central Universities are gratefully acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.041.
| [1] |
A. Persidis, Nat. Biotechnol. 18 (2000) 111-112. DOI:10.1038/71816 |
| [2] |
N.A. Tarunkumar, P.R. Jignesh, Chin. Chem. Lett. 23 (2012) 785-788. DOI:10.1016/j.cclet.2012.05.004 |
| [3] |
T. Conroy, J.T. Guo, N. Elias, et al., J. Med. Chem. 57 (2014) 10557-10563. DOI:10.1021/jm501439w |
| [4] |
P.J. Hotez, B. Pecoul, PLoS Negl. Trop. Dis. 4 (2010) e718. DOI:10.1371/journal.pntd.0000718 |
| [5] |
World Malaria Report 2016, World Health Organization, Geneva, 2016. http://www.who.int/malaria/publications/world-malaria-report-2016/en/.
|
| [6] |
L. Musset, O. Bouchaud, S. Matheron, et al., Microbes Infect. 8 (2006) 2599-2604. DOI:10.1016/j.micinf.2006.07.011 |
| [7] |
A.M. Dondorp, F. Nosten, P. Yi, et al., N. Engl. J. Med. 361 (2009) 455-467. DOI:10.1056/NEJMoa0808859 |
| [8] |
A.M. Dondorp, S. Yeung, L. White, et al., Nat. Rev. Microbiol. 8 (2010) 272-280. DOI:10.1038/nrmicro2331 |
| [9] |
K.T. Andrews, G. Fisher, Skinner-Adams T.S., Int. J. Parasitol.:Drugs Drug Resist. 4 (2014) 95-111. DOI:10.1016/j.ijpddr.2014.02.002 |
| [10] |
S.C. Stolze, E. Deu, F. Kaschani, et al., Chem. Biol. 9 (2012) 1546-1555. |
| [11] |
T.M. Musyoka, A.M. Kanzi, K.A. Lobb, et al., J. Biomol. Struct. Dyn. 35 (2016) 2084-2101. |
| [12] |
C.V. Plowe, J.G. Kublin, O.K. Doumbo, Drug Resist. Updates 1 (1998) 389-396. DOI:10.1016/S1368-7646(98)80014-9 |
| [13] |
K.J. Gresty, K.A. Gray, A. Bobogare, et al., Malar. J. 13 (2014) 402-408. DOI:10.1186/1475-2875-13-402 |
| [14] |
V.T. Metzger, C. Eun, P.M. Kekenes-Huskey, et al., Biophys. J. 107 (2014) 2394-2402. DOI:10.1016/j.bpj.2014.09.039 |
| [15] |
H. Huang, W.Q. Lu, X. Li, et al., Bioorg. Med. Chem. Lett. 22 (2012) 958-962. DOI:10.1016/j.bmcl.2011.12.011 |
| [16] |
Y.Q. Yang, Y. Yu, X.L. Li, et al., J. Med. Chem. 60 (2017) 1994-2005. DOI:10.1021/acs.jmedchem.6b01733 |
| [17] |
N.A. Malmquist, T.A. Moss, S. Mecheri, et al., Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 16708-16713. DOI:10.1073/pnas.1205414109 |
 2019, Vol. 30
2019, Vol. 30 


