b Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China;
c Institute of Applied Chemistry, Jiangxi Academy of Sciences, Nanchang 330096, China
Non-fullerene organic solar cells (NFOSCs) have attracted tremendous attention in recent years [1-7] due to the huge amounts of non-fullerene acceptors developed in recentyears [8-17]. Among them, small molecules based on acceptor-donor-acceptor (A-D-A) structures are widely studied with high power conversion efficiencies (PCEs) over 14% in single junction organic solar cells [18] and over 17% in tandem junction cells [19]. These electron acceptors contain a fused donating backbone that is beneficial for charge delocalization, bulky aromatic side units to prevent aggregation and facilitate phase separation, and two electron-withdrawing end groups in order to tune frontier energy levels. In addition, the pull-push-pull structure in these acceptors can induce intramolecular charge transfer so as to extend absorption spectrum to near-infrared region [20-22]. All these merits make them to lead the research of NFOSCs.
However, these A-D-A type non-fullerene acceptors always have complex chemical structures so that multistep reactions are required to obtain the final molecules. This will severely limit their large scale production and hence large-area devices for industry application. Obviously, it will be an important task to realize high performance non-fullerene acceptors with simple synthetic procedures. For these A-D-A type acceptors, an efficient route to reduce the synthetic steps is to use unfused conjugated backbone to replace fused backbone. For example, Chen and coworkers have developed a novel electron acceptor with an unfused core containing two cyclopentadithiophene (CPDT) moieties and one 2, 5-difluorobenzene (DFB) group [23]. A high PCE of 10.14% in solar cells based on this acceptor could be obtained [24]. Although this molecule still has complex synthetic steps due to the cyclo-core, its outstanding photovoltaic performance demonstrate that non-fused structures can also realize high performance in NFOSCs.
Herein, we intend to develop simple A-D-A electron acceptors with unfused cores for application in NFOSCs. We select 2, 2´-bithiophene (BT) as central core, in which two alkyl side chains, n-hexyl (H) or ethylhexyl (EH), are used to enhance the solubility of the molecules. Side chains were attached to the 3 and 3´ position of BT units to produce appropriate dihedral angel, which is beneficial for creating a good phase separation in organic solar cells. Electron-withdrawing end group 1, 1-dicyanomethylene-3-indanone was used to tune the absorption and energy levels [25]. The resulting molecules BTIC—H and BTIC-EH were found to show near-infrared absorption and aligned energy levels, and their initial application in NFOSCs was studied. Our results demonstrate that these simple unfused electron acceptors have the potential application in NFOSCs.
The synthetic routes of BTIC-H and BTIC-EH were shown in Scheme 1 and the detailed procedures were present in the Supporting information. In general, Starting from 2-bromo-3-alkylthiophene (1-H or 1-EH), the BT derivatives 2-H or 2-EH could be obtained, which were then converted into 3-H or 3-EH with CHO groups. BTIC-H and BTIC-EH were then synthesized via Knoevenagel condensation in the reasonable yield of 80%-90%. Therefore, two new non-fullerene acceptors were obtained via three simple synthetic steps, which is much improved compared to other fused and unfused acceptors [1, 24]. BTIC-H with hexyl side chains perform very poor solubility in chloroform and chlorobenzene (< 2mg/mL), while BTIC-EH with branched EH side chains show good solubility in these solvents (> 20mg/mL).
|
Download:
|
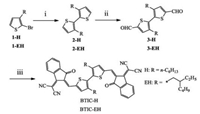
| Scheme 1. The chemical structures of unfused electron acceptors BTIC-H and BTIC-EH and their synthetic procedures. (i) Ni(PPh3)2Cl2, Zn, KI and PPh3 inTHF, refluxed overnight, (ii) TMEDA, n-BuLi, DMF, THF. (iii) Pyridine in chloroform, 40 ℃, 12h. | |
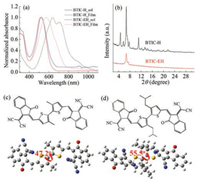
Absorption spectra of the new acceptors were present in Figs. 1a and b. In chloroform solution, BTIC-H and BTIC-EH have identical absorption spectra with the peaks at 520 nm and absorption onset at 625nm. In thin films, BTIC-H has the peak at 638nm with a shoulder at 706 nm and the onset at 825 nm, while BTIC-EH presents the peak at 573 nm and the onset at 757 nm. The significantly red-shifted absorption in thin films indicate that both molecules have twisted backbones in solution, while in thin films they tend to form planar backbones. BTIC-H has the optical band gap (Eg) of 1.50eV, which is much lower than BTIC-EH with Eg of 1.64 eV. This can be attributed to the twisted BT units caused by adjacent alkyl side chains. BT units with hexyl side chains show a dihedral angle of 47.2°, while dihedral angle can increase to 55.5° when using branched EH units (Figs. 1c and d). The large dihedral angle indicates less crystallinity in thin films, explaining relatively large Eg of BTIC-EH. This can also be confirmed by their X-ray diffraction (XRD) patterns, as shown in Fig. 1b. BTIC-H shows multiple diffraction peaks in thin films, while BTIC-EH only has one diffraction peak around 2θ = 6°. From absorption and XRD measurement, we can conclude that although their short conjugated length, these simple unfused electron acceptors can still perform near-infrared absorption spectra that is comparable with other fused electron acceptors.
|
Download:
|
| Fig. 1. (a) Optical absorption spectra in chloroform solution and in thin films, (b) XRD patterns and (c, d) density functional theory calculations based on BTIC-H and BTIC-EH. The dihedral angle between two thiophenes is also included, 47.2° for BTIC-H and 55.5° for BTIC-EH. | |
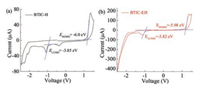
The frontier energy levels ofthe two acceptors in thin films were determined by cyclicvoltammograms (CV) measurement (Fig. 2), in which highest occupied molecular orbital (HOMO) levels ofBTIC-H and BTIC-EH are -6.00 eV and -5.98 eV, and their lowestunoccupied molecular orbital (LUMO) levels are -3.85eV and -3.82eV. Therefore, the different side chains have little impact on their frontier energy levels.Thewideband gap polymerPBDB-T(Fig. S1 in Supporting inforamtion) that will be used as electron donor in this work shows HOMO and LUMO levels of -5.37 eV and -3.57 eV under the same measurement condition [26]. The energy different between donor and the BTIC-based acceptor is close to 0.3 eV that should be enough for exciton dissociation into free charge.
|
Download:
|
| Fig. 2. Cyclic voltammogram of the electron acceptors (a) BTIC-H and (b) BTIC-EH. Potential vs. Fc/Fc+. The calculated HOMO and LUMO levels were also included. | |
The two molecules as electron acceptors were then applied into NFOSCs with the donor polymer PBDB-T. An inverted configuration with ITO/ZnO and MoO3/Ag as electrode was used. The photoactive layers based on PBDB-T:BTIC-H thin films were solution-processed from high boiling point 1, 1, 2, 2-tetrachloroethane (TCE) due to the poor solubility of BTIC-H, while PBDB-T:BTIC-EH thin films can be easily fabricated from CB solution. We perform careful optimization of photoactive layers, including the amount of additive, the ratio of donor to acceptor and the thickness of active layers (Table S1 in Supporting information). It was found that 1, 8-diiodooctane (DIO) as additive can provide the best performance.
PBDB-T:BTIC-H solar cells fabricated from TCE with 1% DIO showed the best PCE of 0.96% with a short-circuit current density (Jsc) of 2.68mA/cm2, open-circuit voltage (Voc) of 0.69 V and fill factor (FF) of 0.52. BTIC-EH with branched side chains as electron acceptor provided the enhanced PCE of 2.4% with a Jsc of 6.33 mA/cm2, Voc of 0.71 V and FF of 0.54. The improved PCE in BTIC-EH based cells is mainly due to the increased photocurrent, which can be further reflected by their external quantum efficiencies (EQEs). As shown in Fig. 3b, both cells have broad photoresponse from 300 nm to 800 nm, in which BTIC-H showed EQEs below 0.1 and BTIC-EH had EQEs close to 0.3. We also selected other conjugated polymers as donor and BTIC-EH as acceptor to fabricated solar cells, in which the PCEs up to 1.79% could be obtained (Fig. S2 and Table S2 in Supporting information).

|
Download:
|
| Fig. 3. (a) J-V characteristics in dark (dashed line) and under white light illumination (solid line). (b) EQE of the optimized solar cells. (c, d) AFM height images (3×3 μm2) of PBDB-T:BTIC-H and PBDB-T:BTIC-EH based thin films. | |
It is very interesting to observe that photoresponse in PBDB-T: BTIC-H cells is up to 800nm (Fig. 3b), which is inconsistent with the absorption spectra ofBTIC-H film with absorption over 800 nm (Fig. 1a). This should be due to different crystalline behavior of BTIC-H in pure and thin films. We speculate that the crystallinity of BTIC-H in blended thin films will be hampered by the donor polymer PBDB-T, resulting in blue-shifted absorption. This can be confirmed by the absorption spectra of PBDB-T:BTIC-H thin film (Fig. S3 in Supporting information) that is consistent with their EQE spectra in solar cells.
We further use atomic force microscopy (AFM) to study the microphase separation of BHJ surface, as shown in Figs. 3c and d. PBDB-T:BTIC-H thin films exhibit large domain size on the surface with a high roughness of RMS = 11.50 nm, while the roughness in PBDB-T:BTIC-EH thin films could reduce to 8.87 nm with decreased domain size. Since small domain size in BHJ thin films can facilitate the exciton diffusion into the interface of donor and acceptor, thus responsible for the high photocurrent. It is worthy mentioned that PCEs of BTIC-EH based cells are still much low compared to other high performance fused-ring based NFOSCs. From AFM images in Fig. 3d, we infer that BTIC-EH still shows strong aggregation tendency in blended thin films, resulting in relatively large domain size compared to other high performance solar cells. Therefore, it will be important to reduce the aggregation of these simple acceptors from chemical design and device optimization in order to improve the photovoltaic performance. For example, the crystallinity of these conjugated molecules can be reduced by using asymmetric structures or introducing furan moiety.
In conclusion, in this work, we successfully developed two simple electron acceptors based on bithiophene as unfused core, in which two side chains, hexyl and ethylhexyl units, were used to tune the crystalline properties. The two molecules show broad absorption in the range of 300nm to 800nm, aligned frontier energy levels and strong aggregation in thin films. The two molecules as electron acceptor were applied into organic solar cells, in which BTIC-H with linear side chains only achieved a PCE of 0.96%. When using branched side chains in BTIC-EH, the corresponding solar cells showed enhanced PCEs up to 2.4%, which can be due to the better microphase separation from less crystalline BTIC-EH. Although the PCE is lag behind fused-ring based electron acceptor, these simple unfused electron acceptors with broad absorption and aligned energy levels demonstrate their great potential application in high performance NFOSCs.
AcknowledgmentsThis study isjointly supported by MOST (No. 2017YFA0204702) and the National Natural Science Foundation of China (Nos. 51773207, 21574138, 51603209, 91633301). This work was further supported by the Strategic Priority Research Program (No. XDB12030200) of the Chinese Academy of Sciences and the Recruitment Program of Global Youth Experts of China.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.09.014.
| [1] |
Y. Lin, J. Wang, Z.G. Zhang, et al., Adv. Mater. 27 (2015) 1170-1174. DOI:10.1002/adma.201404317 |
| [2] |
A. Zhang, C. Li, F. Yang, et al., Angew. Chem. Int. Ed. 56 (2017) 2694-2698. DOI:10.1002/anie.201612090 |
| [3] |
X. Liu, C. Zhang, C. Duan, et al., J. Am. Chem. Soc. 140 (2018) 8934-8943. DOI:10.1021/jacs.8b05038 |
| [4] |
D. Meng, H. Fu, C. Xiao, et al., J. Am. Chem. Soc. 138 (2016) 10184-10190. DOI:10.1021/jacs.6b04368 |
| [5] |
C. Yu, Y. Xu, S. Liang, et al., Chin. Chem. Lett. 29 (2018) 325-327. DOI:10.1016/j.cclet.2017.08.016 |
| [6] |
Y. Guo, A. Zhang, C. Li, W. Li, D. Zhu, Chin. Chem. Lett. 29 (2018) 371-373. DOI:10.1016/j.cclet.2017.08.006 |
| [7] |
W. Wu, G. Zhang, X. Xu, et al., Adv. Funct. Mater. 28 (2018) 1707493. DOI:10.1002/adfm.v28.18 |
| [8] |
C. Tan, J. Gorman, A. Wadsworth, et al., Chem. Commun. 54 (2018) 2966-2969. DOI:10.1039/C7CC09123K |
| [9] |
C. Huang, X. Liao, K. Gao, et al., Chem. Mater. 30 (2018) 5429-5434. DOI:10.1021/acs.chemmater.8b02276 |
| [10] |
X. Li, T. Yan, H. Bin, et al., J. Mater. Chem. A 5 (2017) 22588-22597. DOI:10.1039/C7TA07049G |
| [11] |
F. Yang, C. Li, W. Lai, et al., Mater. Chem. Front. 1 (2017) 1389-1395. DOI:10.1039/C7QM00025A |
| [12] |
H. Lin, S. Chen, H. Hu, et al., Adv. Mater. 28 (2016) 8546-8551. DOI:10.1002/adma.v28.38 |
| [13] |
Y. Liu, C. Mu, K. Jiang, et al., Adv. Mater. 27 (2015) 1015-1020. DOI:10.1002/adma.201404152 |
| [14] |
Y. Duan, X. Xu, Y. Li, Q. Peng, Chin. Chem. Lett. 28 (2017) 2105-2115. DOI:10.1016/j.cclet.2017.08.025 |
| [15] |
J. Miao, B. Meng, J. Liu, L. Wang, Chem. Commun. 54 (2018) 303-306. DOI:10.1039/C7CC08497H |
| [16] |
Y. Duan, X. Xu, Y. Li, Z. Li, Q. Peng, Macromol. Rapid Commun. 38 (2017) 1700405. DOI:10.1002/marc.v38.23 |
| [17] |
H.W. Luo, Z.T. Liu, Chin. Chem. Lett. 27 (2016) 1283-1292. DOI:10.1016/j.cclet.2016.07.003 |
| [18] |
S. Zhang, Y. Qin, J. Zhu, J. Hou, Adv. Mater. 30 (2018) 1800868. DOI:10.1002/adma.v30.20 |
| [19] |
L. Meng, Y. Zhang, X. Wan, et al., Science 361 (2018) 1094-1098. DOI:10.1126/science.aat2612 |
| [20] |
S. Feng, C. Zhang, Y. Liu, et al., Adv. Mater. 29 (2017) 1703527. DOI:10.1002/adma.201703527 |
| [21] |
T. Li, S. Dai, Z. Ke, et al., Adv. Mater. 30 (2018) 1705969. DOI:10.1002/adma.201705969 |
| [22] |
F. Liu, Z. Zhou, C. Zhang, et al., Adv. Mater. 29 (2017) 1606574. DOI:10.1002/adma.201606574 |
| [23] |
W. Lu, J. Kuwabara, T. Kanbara, Poly. Chem. 3 (2012) 3217-3219. DOI:10.1039/c2py20539d |
| [24] |
S. Li, L. Zhan, F. Liu, et al., Adv. Mater. 30 (2018) 1705208. DOI:10.1002/adma.201705208 |
| [25] |
G. Zhang, G. Yang, H. Yan, et al., Adv. Mater. 29 (2017) 1606054. DOI:10.1002/adma.201606054 |
| [26] |
Y. Guo, Y. Liu, Q. Zhu, et al., ACS Appl. Mater. Interfaces 10 (2018) 32454-32461. DOI:10.1021/acsami.8b10955 |
 2019, Vol. 30
2019, Vol. 30 

