b College of Packing and Materials Engineering, Hunan University of Technology, Zhuzhou 412007, China;
c State Key Laboratory of Bioelectronics, Southeast University, Nanjing 210096, China
α-Fetoprotein (AFP) is a glycoprotein belonging to the albumin family [1-3]. The level of AFP concentration may be considered as a sign of some cancers and tumors, such as hepatocellular carcinoma and germinal tumors [4, 5]. In clinical early diagnosis of cancer, screening of tumor biomarkers is one of the general methods applying for immunoassay techniques [6-9]. The electrochemical immunosensors have excellent selectivity, high sensitivity, fast respone and low cost, etc. [10-17]. Highly sensitive and accurate electrochemical immunoassay methods developed for determination of AFP are significant in the clinical screening, diagnosis and monitoring of cancer [18-23].
Recently, molecularly imprinted polymers (MIP), due to their low cost, facile preparation, high selectivity, high sensitivity and stability [24-26], have been paid much more attention on electrochemical sensors widely detecting different molecules ranging from small molecules to biological macromolecules [27-30]. This work is aimed at fabricating a molecular imprinting sensor to detection AFP. Polydopamine (PDA), possessing a large number of catechol functional groups inside [31], has distinctive properties [32, 33], such as facile preparation adhesion [34], hydrophilicity [35], stability, and biocompatibility [36], and is used widely in electrochemical sensor as a MIP.
As a kind of electrode material, polythionine (PTh) has fast charge transfer capacity [37-39]. In this work, PTh film is loaded on the electrode surface to enhance the sensitivity of the sensor. In addition, AuNPs are widely adopted in the design of electrochemical sensors due to their unique properties ofexcellent conductivity, good biocompatibility and a narrow size distribution [23, 40-45].
In this work, the electroactive substance (AuNPs/PTh/GCE) was modified on the surface of GCE. "PDA-AFP" mixture by electro-polymerization DA method was fixed on the modified AuNPs/PTh/GCE surface. After the removal of target molecules, the rebinding properties of target molecules are superior to antibody binding by rebinding of the polymer binding sites. Differential pulse voltammetry (DPV) was used to assess the binding properties of MIP sensors to target molecules.
The applied reagents and apparatus in this paper can be found in Supporting information. Preparation of AuNPs/polythionine (AuNPs/PTh) modified GCE is as follows. The GCE was polished with 0.3 μmol/L and 0.05 μmol/L alumina slurry, followed by ultrasonic washing with DI water, ethanol, DI water, respectively. The bare GCE was characterized in a 10 mmol/L Fe(CN)63-/4- of 10 mmol/L phosphate buffer solution (PBS) to obtain a clean electrode in the potential between -0.6 V to 0.6Vwith a scan rate of 0.05 V/s. The GCE was modified with PTh by electropolymerization. Briefly, the GCE was immersed in 0.5 mmol/L thionine with 10 mmol/L PBS (pH 6.8) and electrodeposited at +1.5 V for 15 min. AuNPs synthesized by reducing HAuCU with sodium citrate according to Fren's method [46]. Then, 10 mL AuNPs solution was dropped on the surface of PTh/GCE to obtain the AuNPs/PTh/ GCE after air-drying at room temperature.
Fabrication of MIP sensor can be described as follows. The AFP- MIP was prepared by electroploymerizing the mixture of DA, AFP and PBS at the surface of AuNPs/PTh/GCE [47]. Briefly, the 5 mmol/L DA, 800 ng/mL AFP and 10 mmol/L PBS were mixed, degassed with nitrogen and incubated for 70 min, then fabricated AuNPs/PTh/GCE was immersed in electroploymerizing mixture and checked by CV (8 cycles, potential range of -0.6 V to 0.6 V, scan rate of 0.02V/s). The electrode was rinsed with PBS and washed 70min in washing solution to remove the AFP template. A non-imprinted 'control' electrode was prepared in the same way without AFP.
The MIP electrochemical immunoassay electrode was immersed respectively in different concentrations of the AFP and the reaction was conducted at 37 ℃ for 50 min, then the electrode was washed with 0.1 mol/L NaOH for 70min to release the combined AFP into solution. The formed MIP immunoassay was respectively measured by DPV in the Fe(CN)63-/4- solution before and after the elution. The value of control constant current of Fe(CN)63-/4- solution was quantitatively decreased with the increase in the concentration of AFP in the solution.
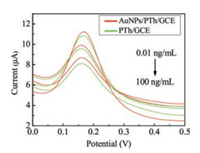
The modified electrode was then characterized. Fig. S1 (Supporting information) is the CV curve of Thi polymerization, Fig. S2 (Supporting information) shows that the different numbers of polymerization cycles have different electrochemical responses. As polymerization cycles of Thi are 10-20 cycles, the peak current increases, while the peak current decreases in 20-30 cycles. The optimum number of polymerization cycles is 20 cycles. The explanation of the above phenomenon is that a low number oflaps will lead to the uneven electrode surface coverage of PTh film, which affects the fixation of materials. However, a high number of laps form a thick film of PTh, resulting in a decrease in electronic transfer rate on the electrode surface. The pH of Thi solution also affects the polymerizing rate and leads to the decrease in the PTh productivity due to the effect of thionine monomer cationic radicals. Thus, the current response exhibits the strongest pH 6.8. The electrochemical response of Fe(CN)63-/4- on the working electrode becomes stronger after modification with PTh and AuNPs properly. Fig. 1 shows that the current of detection AFP at the AuNPs/PTh/GCE is much stronger than those at the PTh/GCE. The results indicate that the AuNPs/PTh/GCE can be incorporated into the AFP-MIP system to improve electron transfer which facilitates the electron transmission between the MIP film and the electrode.
|
Download:
|
| Fig. 1. The DPV curves of the MIP/AuNPs/PTh/GCE (red) and MIP/PTh/GCE (green) for AFP at the concentration of 0.01, 1, 100 ng/mL, respectively. | |
The concentration of target molecules was also studied at 0.1, 0.3, 0.5, 0.8, 1.0, 1.5 μg/mL. As shown in Fig. S3 in Supporting information, after eluting, the peak current of DPV was increased with the increase in the concentration of AFP. While the concentration of AFP was 0.8 μg/mL, the binding sites between DA and AFP reached saturation.
Effect of incubation time was then stuied. From Fig. S4 (Supporting information) it can be seen that the peak current of DPV was increased with the incubation time delay, and no apparent increase was found longer after 70 min. the binding interaction between DA and AFP achieved saturation at 60 min. Thus, incubation time of 70 min for DA-AFP is the best choice.
The PDA electropolymerization cycle was studied at 5, 8, 10, 15, 20 cycles. From Fig. 2, it can be found that the peak current of DPV was decreased with the increase in the number of electro polymerization cycle. The thickness of the PDA will affect the sensitivity and accuracy of the sensor, the PDA film is too thin to completelycover the electrode surface, and too thick to completely elute template molecules, which will affect the detection sensitivity. Thus, the optimal number of polymerization is 8 cycles because the current rapidly drops and is the largest after eluting template molecule. It is indicated that the formed MIP is uniform and the electrochemical response is the strongest under above conditions.

|
Download:
|
| Fig. 2. Effect of DA cycles electropolymerization on DPV peak current of the prepared AFP-MIP (a); 8, 10, 15, and 20 cycles ofDA cycles electropolymerization for the DPV peak current after AFP elution (b). | |
The elution time of the AFP has an effect on the assay result. In Fig. S5 in Supporting information, the peak current of DPV was increased with the increase in elution time, but the current was no longer increased after the elution time exceeded 70 min, indicating that the target molecules were completely eluted from the MIP.
For the effect of adsorption time as shown in Fig. S6 in Supporting information, the corresponding DPV current decreases with the increase in adsorption time and tends to stabilize after 50 min in the AFP-MIP sensing system, indicating that the target molecules have successfully been adsorbed and tend to saturate on the MIP.
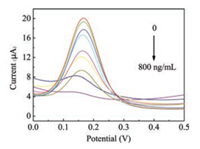
To check the electrochemical performance of the MIP sensor, under the optimal conditions the proposed AFP-MIP/AuNPs/PTh/GCE was successfully prepared, and the measuring results for different concentrations of AFP are showed in Fig. 3. The current of the MIP sensor decreased with the increase in the concentration of AFP. From the results, it can be found that a good linear relationship was presented for AFP (Fig. 4) in the range of 0.001 ng/mL to 800 ng/mL, with the correlation coefficient being 0.9905 and the detection limit being 0.8138 pg/mL.
|
Download:
|
| Fig. 3. DPV responses of the AFP-MIP immunosensor to AFP at the concentration of 0, 0.001, 0.01, 0.1, 0.5, 1, 10, 100, 800 ng/mL. | |

|
Download:
|
| Fig. 4. Relationship between DPV peak current and logarithm value of AFP concentration. | |
Finally, the repeatability, reproducibility, specificityand stability were studied. The repeatability of the MIP sensor of five parallel tests was determined with 800 ng/mL AFP under the optimum conditions. The relative standard deviation (RSD) was 4.9% for AFP. While the reproducibility of the AFP-MIP sensor was investigated by determining the DPV responses of 800ng/mL AFP, and the RSD was 7.0% for six successive assays. To evaluate the specificity of this MIP sensor, four kinds of interfering substances were measured, namely DA, AA, UA, and CEA, respectively. We obtained that no noticeable changes in DPV peak current were detected in presence of 800ng/mL of DA, AA, UA, and CEA, respectively. In addition, to characterize the stability of the proposed MIP sensor, it was stored in a refrigerator at4C for further use. No obvious decrease in peak current was observed in the storage period of first 7 days storage. After storage for 30 days, the variation in the current was less than 21.7%.
To testify and evaluate the accuracy as well as practical application of this method in complex samples, the MIP/PTh/AuNPs/GCE sensor was applied to the detection of AFP in human serum samples (from Hunan Cancer Hospital). For this purpose serum samples were spiked with three different AFP standard concentrations: 100, 200, 400ng/mL. Recoveries of 101.9%, 100.6% and 111.1% with 4.6%, 2.9% and 1.1% for AFP-MIP sensor were obtained, respectively. The DPV electrochemical measurement results of the samples spiked with AFP standard solution are shown in Table S1 (Supporting information). These results demonstrate the practicability of the MIP sensor in human serum sample successfully.
In conclusion, the AFP-MIP immunosensors using PTh/AuNPs/GCE as substance, DA as imprinting monomer and AFP as target molecular, were successfully fabricated. In this study, the PTh/ AuNPs provided a highly conductive and stabile substrate for the imprinting film. In addition, the MIP/PTh/AuNPs/GCE sensor presented good rebinding of AFP to the selective cavities of MIP and high selectivity to the AFP, respectively. Therefore, the application of this AFP-MIP as a recognition element for electrochemical sensors demonstrates a promising alternative for the determination of AFP. The satisfactory results demonstrated that the AFP-MIP immunosensor has a great potential for the detection of AFP in human serum sample.
AcknowledgmentsWe acknowledge the National Natural Science Foundation of China (Nos. 61471168, 61571187), China Postdoctoral Science Foundation (No. 2016T90403), Postdoctoral Science Foundation ofJiangsu Province (No. 1601021A), the Natural Science Foundation of Hunan Province (No. 2017JJ209), and Hunan Key Research Project (No. 2017SK2174) for the financial supports.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.07.011.
| [1] |
J.H. Schieving, M. Vries, J.M.G. Vugt, et al., Eur. J. Paediatr. Neurol. 18 (2014) 243-248. DOI:10.1016/j.ejpn.2013.09.003 |
| [2] |
E.N. Debruyne, J.R. Delanghe, Clin. Chim. Acta 395 (2008) 19-26. DOI:10.1016/j.cca.2008.05.010 |
| [3] |
Y.L. Wang, D. Wu, Y. Zhang, et al., RSC Adv. 5 (2015) 56583-56589. DOI:10.1039/C5RA07547E |
| [4] |
L. Liu, L.H. Tian, G.H. Zhao, et al., Anal. Chim. Acta 986 (2017) 138-144. DOI:10.1016/j.aca.2017.07.025 |
| [5] |
L. Li, L.N. Zhang, J.H. Yu, et al., Biosens. Bioelectron. 71 (2015) 108-114. DOI:10.1016/j.bios.2015.04.032 |
| [6] |
B.E. Burcu, S.M. kemal, Talanta 132 (2015) 162-174. DOI:10.1016/j.talanta.2014.08.063 |
| [7] |
Y. Wei, Y. Li, N. Li, et al., Biosens. Bioelectron. 79 (2016) 482-487. DOI:10.1016/j.bios.2015.12.082 |
| [8] |
J. Kirsch, C. Siltanen, Q. Zhou, A. Revzin, A. Simonian, Chem. Soc. Rev. 42 (2013) 8733-8768. DOI:10.1039/c3cs60141b |
| [9] |
S. Sharma, R. Raghav, R. O'Kennedy, S. Srivastava, Enzyme Microb. Technol. 89 (2016) 15-30. DOI:10.1016/j.enzmictec.2016.03.002 |
| [10] |
X.F. Tan, L.H. Zhang, H.G. Li, et al., J. Biomed. Nanotechnol. 13 (2017) 973-979. DOI:10.1166/jbn.2017.2412 |
| [11] |
F.J. Zhao, L.L. Cao, Y.B. Liang, et al., J. Biomed. Nanotechnol. 13 (2017) 1300-1308. DOI:10.1166/jbn.2017.2415 |
| [12] |
S. Khan, Z.A. Ansari, O.Y. Alothman, H. Fouad, S.G. Ansari, Nanosci. Nanotechnol. Lett. 9 (2017) 1656-1664. DOI:10.1166/nnl.2017.2544 |
| [13] |
T.T. Song, W. Wang, L.L. Meng, et al., Chin. Chem. Lett. 28 (2017) 226-230. DOI:10.1016/j.cclet.2016.07.021 |
| [14] |
N. Liu, Z. Ma, Biosens. Bioelectron. 51 (2014) 184-190. DOI:10.1016/j.bios.2013.07.051 |
| [15] |
J. Zou, L.L. Huang, X.Y. Jiang, F.P. Jiao, J.G. Yu, Nanosci. Nanotechnol. Lett. 9 (2017) 1700-1707. DOI:10.1166/nnl.2017.2525 |
| [16] |
N. Wang, H.X. Dai, D.L. Wang, et al., Nanosci. Nanotechnol. Lett. 8 (2016) 581-585. DOI:10.1166/nnl.2016.2165 |
| [17] |
Y. Ma, Y.M. Yu, M. Xu, et al., Nanosci. Nanotechnol. Lett. 8 (2016) 592-598. DOI:10.1166/nnl.2016.2037 |
| [18] |
Y.L. Niu, T. Yang, S.S. Ma, et al., Biosens. Bioelectron. 92 (2017) 1-7. DOI:10.1016/j.bios.2017.01.069 |
| [19] |
Y. Liu, Y. Deng, H.M. Dong, K.K. Liu, N.Y. He, Sci. China Chem. 60 (2017) 1-9. |
| [20] |
N. Alizadeh, A. Salimi, R. Hallaj, J. Electroanal. Chem. 811 (2018) 8-15. DOI:10.1016/j.jelechem.2017.12.080 |
| [21] |
G. Li, S. Li, Z. Wang, et al., Anal. Biochem. 547 (2018) 37-44. DOI:10.1016/j.ab.2018.02.012 |
| [22] |
F. Peng, M. Chu, J. Sun, et al., J. Electroanal. Chem. 814 (2018) 52-58. DOI:10.1016/j.jelechem.2017.12.076 |
| [23] |
Y.X. Lai, L.J. Wang, Y. Liu, et al., J. Biomed. Nanotechnol. 14 (2018) 44-65. DOI:10.1166/jbn.2018.2505 |
| [24] |
L. Chen, S. Xu, J. Li, Chem. Soc. Rev. 40 (2011) 2922-2942. DOI:10.1039/c0cs00084a |
| [25] |
J. Erdőssy, V. Horváth, A. Yarman, F.W. Scheller, R.E. Gyurcsányi, TrAC Trends Anal. Chem. 79 (2016) 179-190. DOI:10.1016/j.trac.2015.12.018 |
| [26] |
R. Gui, H. Jin, H. Guo, Z. Wang, Biosens. Bioelectron. 100 (2018) 56-70. DOI:10.1016/j.bios.2017.08.058 |
| [27] |
X. Kan, Z.L. Xing, A.H. Zhu, et al., Sensor. Actuators B Chem. 168 (2012) 395-401. DOI:10.1016/j.snb.2012.04.043 |
| [28] |
M. Jiang, M. Braiek, A. Florea, et al., Toxins 7 (2015) 3540-3553. DOI:10.3390/toxins7093540 |
| [29] |
N.W. Turner, C.W. Jeans, K.R. Brain, et al., Biotechnol. Prog. 22 (2006) 1474-1489. DOI:10.1002/btpr.v22:6 |
| [30] |
Y. Liu, L. Zhu, Y. Hu, et al., Food Chem. 221 (2017) 1128-1134. DOI:10.1016/j.foodchem.2016.11.047 |
| [31] |
Y.F. Zhang, M.L. Qi, R.N. Fu, Chin. Chem. Lett. 27 (2016) 88-90. DOI:10.1016/j.cclet.2015.05.054 |
| [32] |
H. Li, W.W. Du, L. Jiao, et al., J. Biomed. Nanotechnol. 13 (2017) 1235-1242. DOI:10.1166/jbn.2017.2416 |
| [33] |
Y. Liu, K. Ai, L. Lu, Chem. Rev. 114 (2014) 5057-5115. DOI:10.1021/cr400407a |
| [34] |
Z.Y. Xi, Y.Y. Xu, L.P. Zhu, Y. Wang, B.K. Zhu, J. Membrane Sci. 327 (2009) 244-253. DOI:10.1016/j.memsci.2008.11.037 |
| [35] |
S.H. Ku, J. Ryu, S.K. Hong, H. Lee, C.B. Park, Biomaterials 31 (2010) 2535-2541. DOI:10.1016/j.biomaterials.2009.12.020 |
| [36] |
X.M. Kang, W.H. Cai, S. Zhang, S.X. Cui, Poly. Chem. 8 (2017) 860-864. DOI:10.1039/C6PY02005D |
| [37] |
M. Chen, C.F. Zhao, Q.S. Xu, et al., Anal. Methods 7 (2015) 10339-10344. DOI:10.1039/C5AY02580J |
| [38] |
S. Weng, Q. Liu, C. Zhao, et al., Sensor. Actuat. B-Chem. 216 (2015) 307-315. |
| [39] |
C.F. Zhao, Z.Q. Jiang, X.H. Cai, et al., J. Electroanal. Chem. 748 (2015) 16-22. DOI:10.1016/j.jelechem.2015.04.025 |
| [40] |
T. Wu, H.T. Wang, B. Shen, et al., Chin. Chem. Lett. 27 (2016) 745-748. DOI:10.1016/j.cclet.2016.01.059 |
| [41] |
M. Taei, F. Hasanpour, M. Dinari, et al., Chin. Chem. Lett. 28 (2017) 240-247. DOI:10.1016/j.cclet.2016.07.025 |
| [42] |
P. Jolly, P. Zhurauski, J.L. Hammond, et al., Sensor. Actuat. B-Chem. 251 (2017) 637-643. |
| [43] |
Y. Liu, K.K. Liu, H.M. Dong, et al., Nanosci. Nanotechnol. Lett. 8 (2016) 785-790. DOI:10.1166/nnl.2016.2264 |
| [44] |
F. Keshvan, M. Bahram, K. Farhadi, Chin. Chem. Lett. 27 (2016) 847-851. DOI:10.1016/j.cclet.2016.01.022 |
| [45] |
Y. Liu, Y.X. Lai, G.J. Yang, et al., J. Biomed. Nanotechnol. 13 (2017) 1253-1259. DOI:10.1166/jbn.2017.2424 |
| [46] |
G. Frens, Z. Kolloid, J. Thukevich, et al., Nat. Phys. Sci. 241 (1973) 20-22. DOI:10.1038/physci241020a0 |
| [47] |
P. Jolly, V. Tamboli, R. Harniman, et al., Biosens. Bioelectron. 75 (2016) 188-195. DOI:10.1016/j.bios.2015.08.043 |
 2019, Vol. 30
2019, Vol. 30 

