b Department of Polymer/Nano Science and Technology, Chonbuk National University, Jeonju 561756, Republic of Korea;
c National Center for Nanoscience and Technology(NCNST), Beijing 100190, China
The exploitation of one-dimensional (1D) conductive micro/ nanostructures is expected to lead to a novel generation of miniaturized processors and sensors which are different from those of current silicon-based electronics [1-3]. Therefore, fabrication of supramolecular conducting nanofibers, which might enable the electrical connection of micro/nanometer scale electronic structures, has been received extensive attention [4, 5]. Self-assembly of organic π-conjugated molecules into low molecular mass organic gelators (LMOGs) offers a simple and powerful approach to the spontaneous formation of micro/nanofibers [6-8]. On the one hand, the formation of supramolecular micro/nanofibers occurs in a selfassembled bottom-up approach through non-covalent forces, such as π–π stacking, hydrogen bonding or hydrophobic interactions [9, 10]. On the otherhand, gelators as soft materials can be deposited on any surface, and the solvent immobilized upon gel formation can be removed by evaporation to leave the active micro/nanostructures of xerogels, which provide the necessary processability and flexibility for practical applications. Intense research has been devoted to build the family of conductive architectures from organogelators [11].
Tetrathiafulvalene (TTF) is known as an organic conductive material, high electron conductivity of which is originated from their π-stacked columnar structures in its single crystals [12-16]. Recently, TTF derivatives have been intensely studied with a focus on fibrous gelators for electrically conducting materials. The micro/ nanofibers from 1D columnar structure would furnish key materials for advanced nanoscience and electronics [17, 18]. Previous reports indicate that the use of intermolecular noncovalent interactions via a gelation process to construct TTF-based conducting fibers is very appealing to construct supramolecular 1D micro/nanofibers. In the gel state, TTF molecules aggregate and immobilize the surrounding solvent, offering a good way to form conducting TTF-based micro/nanofibers with mixed valence states. Electrical conduction in these TTF-based fibers occurs because of the stacking between the π-surfaces of the functional unit as well as the network of short S⋯S contacts between the 'sides' of the molecules, and the existence of an unfilled state upon oxidation of TTF. These studies validate that a low molecular-weight gel strategy shows potential for the construction 1D columnar structure of TTF [19]. The mixed-valence state of the TTF fibrous structures upon doping further generates the conductivity, potentially providing soft materials for organic electronics.
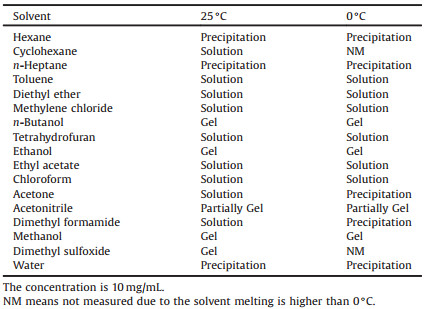
We are interested in supramolecular interactions and their selfassembled structures of TTF derivatives [20-24]. Through extended TTF based π-π interactions and hydrophobic interactions. TTF derivatives show liquid crystalline properties, which are promising active materials for organic electronics. Moreover, the particular interest in building 1D conductive micro/nanostructures and understanding cooperative intermolecular interactions involved in self-assembly process promote us to prepare the amphiphilic TTF derivative (am-TTF) (Fig. 1) [24]. The TTF core is fused with a naphenyl group to increase the π-π stacking, and the long hydrophobic long alkyl chains and tri(ethylene glycol) chains are employed to impart the van der Waals forces. The am-TTF molecule could self-assemble into columnar structure, which further forms fibrous structures with various diameters in the micrometer length scale through the cooperative multiple intermolecular interactions. Herein, we report the self-assembly behavior of am-TTF in solution. In some polar solvent, it can form organogel with micro/ nanofibrous structures, which provide highly-ordered columnar structures. Moreover, the oxidized am-TTF organogel forms charge transfer (CT) state and shows a semiconducting feature. The organic fibrous structures of am-TTF show great potential for organic electronics.
|
Download:
|
| Fig. 1. Schematic synthetic route for am-TTF. i: DCC, DMAP, room temperature, CH2Cl2; ii: Hg(OAc)2, CH2Cl2, iii: P(OEt)3, 120 ℃. | |
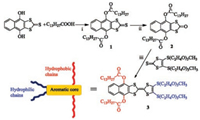
The am-TTF was synthesized according to the route as shown in Fig. 1, and the molecular structure was characterized by NMR, MS, and elementary analysis (details see Supporting information) [24]. The am-TTF was expected to be gelation in some organic solvents with the aid of the cooperative multiple intermolecular interactions, including π-π stacking, hydrophobic interactions, and S⋯S interactions. In general, the am-TTF was dissolved in most organic solvent, such as toluene, diethyl ether and methylene chloride, forming solution with the concentration of 10 mg/mL (Table 1). In none-polar solvent, hexane and n-heptane, compound was not soluble, even under heating. In strong polar solvent, such as dimethyl sulfoxide (DMSO), methanol (MeOH), ethanol and n-butanol, the am-TTF was not dissolve at room temperature, but heating allowed the dissolution of am-TTF in above polar solvent, such as in DMSO (Fig. 2a). Subsequently, yellow gel formed with the concentration of 10 mg/mL upon cooling (Fig. 2b). Interestingly, in DMF, am-TTF formed solution at 25 ℃ and precipitation at 0 ℃. Transparent yellow organogels were stable for several days when am-TTF was dissolved in DMSO and MeOH by heating to around 50 ℃, then air cooled and left to stand. The rheological property of the am-TTF gel was further investigated by dynamic time sweep rheological experiments. As shown in Fig. 2c, the magnitude of G' is much higher than that of G'', revealing that the mechanical properties of the sample were dominated by the solid phase rather than the liquid phase, i.e., low-mass am-TTF based organogels.
|
Download:
|
| Fig. 2. Tuning the formation of solution (a) from heating to the formation of gel (b) upon cooling to room temperature of am-TTF in DMSO with the concentration of 10 mg/mL. (c) Rheological characterization of am-TTF gel. (d) The cyclic voltammetry measurement of am-TTF in a solution of Bu4NBF4 (0.1 mol/L) in water-free dichloromethane with a scan rate of 100 mV/s at room temperature. (e) The UV spectra for monitoring am-TTF oxidation in DMSO. (f) The am-TTF gel in DMSO with 10 mg/mL at 25 ℃ oxidized by FeCl3 solution, and the resultant oxidized am-TTF gel (g). | |
|
|
Table 1 Gelation properties of am-TTF in selected organic solvents at 25 ℃ and 0 ℃, respectively. |
In order to evaluate electrochemical properties, the cyclic voltammetry (CV) measurements were carried out in a dry dichloromethane solution of Bu4NBF4 (0.1 mol/L) with a scan rate of 100 mV/s at room temperature. As shown in Fig. 2d, two irreversible single-electron oxidation peaks at ~0.89 V and 1.20 V were observed, indicating the formation of radical cations and dications of am-TTF, respectively. Due to the introduction of electron-withdrawing ester into TTF core structure, both oxidation potentials of am-TTF are higher than those of TTF [24]. As expectation, the am-TTF could be oxidized by FeCl3 in dichloromethane solution (ca. 2 ×10-5 mol/L). This process was achieved by a stepwise addition of FeCl3, and monitored by UV–vis absorption spectral change (Fig. 2e). The results indicated that am-TTF was chemically oxidized to its radical (am-TTF+) by FeCl3, which was confirmed by the new absorption bands at 469 and 807 nm. The absorptions at 469 was assigned to an intramolecular charge transfer of radical cation, am-TTF+, while the absorption band at 807 nm was due to an intermolecular electron transfer of the π-dimer of am-TTF dications [25]. Therefore, FeCl3 gel (DMSO) was utilized for the oxidation of the am-TTF gel. First, the FeCl3 solution was carefully put on top of am-TTF gelator (Fig. 2f). The am-TTF gel was oxidized in 2 h with the gel color changing from yellow to brown, indicating formation of the charge transfer state (Fig. 2g).
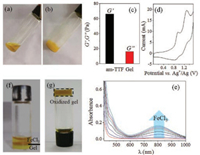
In order to further study the gel, the scanning electron microscope (SEM) was used to measure the morphologies of the am-TTF based gel in DMSO. As shown in Fig. 3a, the am-TTF gel showed highly ordered micro/nanofibers to form fibrous network.
|
Download:
|
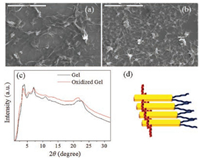
| Fig. 3. SEM images of am-TTF gel (a) and oxidized am-TTF gel (b) in DMSO with 10 mg/mL at 25 ℃. (c) The XRD profiles of am-TTF gel and oxidized am-TTF gel in DMSO with 10 mg/mL at 25 ℃. (d) The schematic illustration of am-TTF molecular packing in gel state. | |
The width was about 1.9 ± 0.8 mm, and the length was more than 50 mm. In order to get the insight, we measured the X-ray diffraction (XRD) patterns of the supramolecular structure of amTTF gel. As shown in Fig. 3c, there were many sharp reflection peaks appeared in both low (3.62°, 4.05°, 5.70°) and wide angle regions (21.71°). These results indicated that the asymmetric TTF molecules formed highly ordered columnar structures. The d-spacing calculated from diffraction at 3.62° was 2.43 nm, which may suggest the side by side molecular packing mode. Moreover, the diffraction at 21.71° indicated the π-π stacking (0.41 nm) of am-TTF self-assembled structures [26]. Together with the nanophase separation between TTF and hydrophobic alkyl and hydrophilic tri(ethylene oxide) tails, the am-TTF may self-assemble into one-dimensional columnar structures, which was shown in Fig. 3d.
The formation of CT complex and conductivity of am-TTF gel was expected to be achieved by oxidation. The oxidized am-TTF gel was firstly characterized by time-dependent IR spectra. As shown in Fig. 4, the changes in the IR spectra indicated the formation of the CT complex. A broad CT band of the mixed valence state appeared in the IR region over 1700 cm-1. Furthermore, there was a new sharp band at 1396 cm-1 upon oxidation due to the coupling of a conduction electron with the vibrational mode of the TTF moiety [27, 28]. This band became broader and broader with the formation of a mixed-valence state. No significant change was observed after 23 days [28]. These results were in good agreement with the previous reports on iodine doped films having TTF moieties. The formation of am-TTF based CT complex was further characterized by SEM and XRD. The oxidized am-TTF gel showed similar morphologies with am-TTF gel as shown in Fig. 3b. In addition, the XRD measurements still exhibited the highly ordered columnar structure with similar with strong diffractions at 3.62°, 5.34°, 5.55°, 7.12°, 10.77°, 21.42°, similar to XRD patterns of am-TTF gel (Fig. 3c) [29]. These results indicated that molecular assembled structures were not disturbed by oxidation.

|
Download:
|
| Fig. 4. FT-IR of am-TTF oxidized by FeCl3 in 23 days and the conductivity of oxidized am-TTF gel after 23 days. | |
Finally, the xerogels from as-prepared gel of am-TTF molecules were prepared for the measurement of conductivity by four-probe method at room temperature [30, 31]. The previous report revealed that the conductivity increased with the oxidizing time until it reached a plateau value, which corresponded to the formation of the full CT state and the subsequent the formation of mixed valence state and could be announced by the IR spectra [28]. Therefore, we measured the conductivity at day 23 after oxidation. As shown in Fig. 4, the conductivity of oxidized am-TTF based gel was up to 6.27 × 10-4 S/cm. However, there were no signals detected by this four-probe method for the am-TTF based xerogels without oxidation, indicating the electrical conductivity was smaller than 10-7 S/cm. Therefore, it could be concluded that the formation of the mixed-valence state was essential to acquire the electric conducting TTF assemblies.
In summary, we described the self-assembly and conductive properties of supramolecular organogels based on am-TTF molecules. The XRD measurements indicated the formation of highly-ordered columnar structures, and SEM observation provided clear evidence for the self-assembled micro/nanofibers structures based on am-TTF molecules. The organogels can be oxidized and form charge transfer state, remaining the micronanofibrous structures with the semiconducting values of about 10-4 S/cm. This may lead to the fabrication of self-assembled 1D solid conductive fibers.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 61106068), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis (No. 130028831).
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.07.001.
| [1] |
H.C. Chang, C.L. Liu, W.C. Chen, Adv. Funct. Mater. 23 (2013) 4960-4968. DOI:10.1002/adfm.v23.39 |
| [2] |
D.A. Stone, A.S. Tayi, J.E. Goldberger, L.C. Palmer, S.I. Stupp, Chem. Commun. 47 (2011) 5702-5704. DOI:10.1039/c1cc10809c |
| [3] |
H.P. Cong, J.F. Chen, S.H. Yu, Chem. Soc. Rev. 43 (2014) 7295-7325. DOI:10.1039/C4CS00181H |
| [4] |
H. Cui, A.G. Cheetham, E.T. Pashuck, S.I. Stupp, J. Am. Chem. Soc. 136 (2014) 12461-12468. DOI:10.1021/ja507051w |
| [5] |
X. Yan, S. Li, T.R. Cook, et al., J. Am. Chem. Soc. 135 (2013) 14036-14039. DOI:10.1021/ja406877b |
| [6] |
M. Wang, I.V. Anoshkin, A.G. Nasibulin, et al., Adv. Mater. 25 (2013) 2428-2432. DOI:10.1002/adma.v25.17 |
| [7] |
M. Hasegawa, M. Iyoda, Chem. Soc. Rev. 39 (2010) 2420-2427. DOI:10.1039/b909347h |
| [8] |
N.X. Zhang, P. Lin, J.J. Li, et al., Chin. Chem. Lett. 29 (2018) 276-280. DOI:10.1016/j.cclet.2017.06.005 |
| [9] |
Z.Y. Li, Y. Zhang, C.W. Zhang, et al., J. Am. Chem. Soc. 136 (2014) 8577-8589. DOI:10.1021/ja413047r |
| [10] |
W. Zheng, L.J. Chen, G. Yang, et al., J. Am. Chem. Soc. 138 (2016) 4927-4937. DOI:10.1021/jacs.6b01089 |
| [11] |
Y. Si, L. Wang, X. Wang, et al., Adv. Mater. 29 (2017)1700339.
|
| [12] |
S.L. Cai, Y.B. Zhang, A.B. Pun, et al., Chem. Sci. 5 (2014) 4693-4700. DOI:10.1039/C4SC02593H |
| [13] |
T.C. Narayan, T. Miyakai, S. Seki, M. Dinca, J. Am. Chem. Soc. 134 (2012) 12932-12935. DOI:10.1021/ja3059827 |
| [14] |
E. Coronado, J.R. Galan-Mascaros, C.J. Gomez-Garcia, V. Laukhin, Nature 408 (2000) 447-449. DOI:10.1038/35044035 |
| [15] |
G.Q. Liang, Z.B. Zhang, H.Q. Li, Y.P. Wang, C.Y. Xian, Chin. Chem. Lett. 25 (2014) 579-582. DOI:10.1016/j.cclet.2014.01.018 |
| [16] |
Y.G. Zhen, H.L. Dong, L. Jiang, W.P. Hu, Chin. Chem. Lett. 27 (2016) 1330-1338. DOI:10.1016/j.cclet.2016.06.023 |
| [17] |
A.M. Amacher, J. Puigmarti-Luis, Y. Geng, et al., Chem. Commun. 51 (2015) 15063-15066. DOI:10.1039/C5CC06819C |
| [18] |
Y. Liu, Z. Yin, L. Jin, B. Yin, Dyes Pigm. 140 (2017) 500-511. DOI:10.1016/j.dyepig.2016.10.025 |
| [19] |
C. Wang, D.Q. Zhang, D.B. Zhu, J. Am. Chem. Soc. 127 (2005) 16372-16373. DOI:10.1021/ja055800u |
| [20] |
L. Wang, S.J. Park, S.H. Lee, et al., Chem. Mater. 21 (2009) 3838-3847. DOI:10.1021/cm901381p |
| [21] |
D.Y. Kim, L. Wang, Y. Cao, et al., J. Mater. Chem. 22 (2012) 16382-16389. DOI:10.1039/c2jm32070c |
| [22] |
L. Wang, H. Cho, S.H. Lee, et al., J. Mater. Chem. 21 (2011) 60-64. DOI:10.1039/C0JM02357D |
| [23] |
L. Wang, K.U. Jeong, M.H. Lee, J. Mater. Chem. 18 (2008) 2657-2659. DOI:10.1039/b805023f |
| [24] |
N. Kim, L. Wang, D.Y. Kim, et al., Soft Matter 8 (2012) 9183-9192. DOI:10.1039/c2sm26458g |
| [25] |
H. Spanggaard, J. Prehn, M.B. Nielsen, et al., J. Am. Chem. Soc. 122 (2000) 9486-9494. DOI:10.1021/ja000537c |
| [26] |
M. Hasegawa, H. Enozawa, Y. Kawabata, M. Iyoda, J. Am. Chem. Soc. 129 (2007) 3072-3073. DOI:10.1021/ja069025+ |
| [27] |
J. Puigmarti-Luis, V. Laukhin, A.P. del Pino, et al., Angew. Chem. Int. Ed. 46 (2007) 238-241. DOI:10.1002/(ISSN)1521-3773 |
| [28] |
T. Kitamura, S. Nakaso, N. Mizoshita, et al., J. Am. Chem. Soc. 127 (2005) 14769-14775. DOI:10.1021/ja053496z |
| [29] |
Y. Tatewaki, T. Hatanaka, R. Tsunashima, et al., Chem.-Asian J. 4 (2009) 1474-1479. DOI:10.1002/asia.v4:9 |
| [30] |
Y. Kobayashi, M. Hasegawa, H. Enozawa, M. Iyoda, Chem. Lett. 36 (2007) 720-721. DOI:10.1246/cl.2007.720 |
| [31] |
X.J. Wang, L.B. Xing, W.N. Cao, et al., Langmuir 27 (2011) 774-781. DOI:10.1021/la103686n |
 2019, Vol. 30
2019, Vol. 30