Ternary Ⅰ-Ⅲ-Ⅵ2 Quantum dots (QDs) have gained more and more attention due to their excellent biocompatibility, optical properties, and large absorption coefficient [1-5]. Among them, AgInS2 QDs are a kind of direct-band-gap semiconductor and have adjustable optical band gap in the visible-to-near-infrared region. AgInS2 QDs with different structure, tunable spectral regions and small particle radius (less than 5.5 nm) can be synthesized by changing the ratio of precursors, reaction time or coating shell [6]. So far, many methods have been reported to synthesize AgInS2 QDs, such as hot injection [7, 8], heating-up [9, 10], solvothermal [11], hydrothermal and microwave irradiation approach [12, 13]. During the synthesis of colloidal QDs, both size and shape of the QDs are affected by temperature because that the formation of nanocrystals requires adequate amount of thermal energy [6]. However, most of the methods above are based on batch reactors, leading to uneven distribution of temperature, bringing poor homogeneity to the nanomaterials and low reproducibility between batches. Therefore, it is important to accurately control the temperature and realize the homogeneous nanomaterials synthesis. For AgInS2 QDs, it is necessary to study the temperature effect on fluorescence properties so as to provide a theoretical basis for high-quality QDs synthesis.
In recent years, owing to their fast mass/heat transfer, effective mixture of reagents, good reproducibility and better control of reaction conditions, microfluidic reactors are showing unique advantages over batch reactors, and have been more and more used in the synthesis of nanomaterials and related mechanism exploration [14-17]. The development of microreactors is from the initial single-phase continuous flow to multiphase segmented flow. Continuous flow systems have been successfully applied to synthesize various materials, including metal nanomaterials (Au, Ag, Cu, among others), semiconductors (Ⅱ-Ⅵ and Ⅲ-Ⅴ compounds) [18]. However, in continuous flows, the linear velocity of the liquid distributes unevenly in the same cross section, making it difficult to control the reaction time. Droplet microreactors make a homogeneous velocity, and reactants in droplets mix quickly, avoiding direct contact with channel wall [19]. Shu et al. synthesized different-sized water-soluble Ag2S QDs that were NIR-emitting and visible-emitting at different temperatures in droplet microreactors [20]. In addition, when integrated with specific functional units, researchers can realize in situ monitor and accurate reaction control in droplet microreactors [20-23]. Yashina et al. designed a two-stage droplet microfluidic platform integrated with a real-time optical detection system for the synthesis of oilsoluble CuInS2/ZnS NCs [15].
In this work, we designed a temperature-controllable droplet microreactor with more accurate and convenient temperature control than that in batch reactors and coupled with an optical fiber optic spectrometer to monitor the fluorescence spectra in situ. The microfluidic chip was fabricated using soft lithography method. First, the structure with a flow-focusing region and a temperature-controllable reaction region was fabricated on a silicon wafer with 50 μm thick SU8 2050 photoresist. After that, the PDMS mixture of a 10:1 precursor/curing agent was poured onto the silicon master with about thickness of 5 mm. After curing at 75 ℃ for 4 h, PDMS was peeled off from the master, and chip inlets and outlets holes were punched with a metal pipe. The temperature-controllable region was realized by a conductive area on ITO glass. A piece of ITO glass with designed structure was fabricated by soft lithography method and covered with AZ1500 photoresist. Then, 9 mol/L HCl solution was used to etch the ITO glass, leaving the photoresist covered area unetched, and then the photoresist was washed off with ethanol solution. Finally, the PDMS was bonded to the ITO glass with designed structure by O2 plasma treatment to form a close chip, and the chip was baked at 120 ℃ for more than 20 h to make the microchannels hydrophobic. A homemade temperature-controlling device was used to accurately control the local heating of the chip. Copper wires were attached onto the ITO glass substrate with high purity silver paint. A PT-100 micro-thermistor (FMC 2101) located under the microfluidic chip was used as temperature sensor.
In the upstream region of the chip, two immiscible fluids were pumped into the microchannel by syringe pumps (pump 11 Pico Plus, Harvard Apparatus, USA). One of them was a fluorinated oil continuous phase, the other was a disperse phase containing 5 mmol/L Na2S precursor solution and a mixture of Ag/In precursors solution (2.5 mmol/L AgNO3 and 17.5 mmol/L In(NO3)3 fully mixed with 150 μL MPA, and 0.25 mol/L NaOH was used to adjust the solution pH to about 7.0). The water-in-oil (W/O) droplets were formed at the flow-focusing structure. The size of the droplets could be adjusted by changing the velocity ratio of the continuous phase to the disperse phase. The flow behavior of droplets was observed under an inverted microscope (TE2000-U, Nikon Corp, Japan) equipped with a CCD camera (Retiga 2000R, Qimaging Corp, Canada).
In the downstream region of the chip, once the droplets came into the heating area, the reaction inside the droplets began. And when droplets flowing through the local reaction region, reaction conditions were changed by setting the temperatures of the reaction zone from 30 ℃ to 70 ℃. A 5-hour continuous synthesis was operated to investigate the stability of the chip, and five chips were used under the same experimental conditions to investigate the reproducibility. A portable fiber optic spectrometer (QE65000, Ocean Optics, USA) coupled with the microscope was employed to monitor the fluorescence spectra of the products in situ.
The schematic diagram of the droplet microfluidic chip was shown in Fig. 1a. Two dispersed phases (one was a mixed solution of Ag/In precursors, the other was a solution of Na2S precursor) presented a stable laminar flow before arriving at the flowfocusing structure. After that, W/O droplets were formed by the shear of the continuous phase (fluorinated oil). The droplet microfluidic chip allowed for the generation of monodisperse droplets of different sizes by adjusting the velocity ratio of the continuous phase to the disperse phase (Fig. S1 in Supporting information). Moreover, the droplets flowed through the reaction region without interfusing with each other at 80 ℃ (Fig. S1) and even a higher temperature (data not shown), indicating that droplet microreactors were stable enough for the synthesis of AgInS2 QDs under high-temperature conditions.

|
Download:
|
| Fig. 1. (a) Schematic diagram of the droplet microfluidic chip. (b) Schematic diagram of water-soluble AgInS2 QDs synthesis in a droplet. (c, d) Optical images of droplets in the flow-focusing and reaction region at 50 ℃. All the scale bars are 200 μm. | |
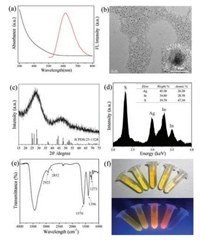
As the droplets flowed through the reaction region, the synthesis of QDs within the droplets was continuously carried out. In this process, MPA acted as surface ligands to stabilize and control the QDs growth. Online fluorescence spectra of AgInS2 QDs from individual droplets was obtained in situ with a portable fiber optic spectrometer. Fig. 2a shows the fluorescence and absorption spectra of AgInS2 QDs synthesized in droplets at 50 ℃. QDs had a fluorescence peak at 615 nm and full width at half-maximum (FWHM) of 110 nm demonstrating typical characteristics of ternary QDs [24]. In addition, QDs synthesized in droplet microreactors showed a slightly narrower FWHM and a more uniform size distribution than that in the flask (Fig. S2 in Supporting information), showing better ability in size control. Fig. S3 (Supporting information) showed fluorescence spectra of QDs at different positions of the microchannel. The peak intensity increased with the residence time and tended to be stable, and the peak position remained unchanged. The residence time of the droplets in the whole chip was less than 1 min, so the reaction time was much shorter than that in the flask (1 h). The size of the QDs was about (3.9 ± 0.6) nm, with the narrow size distribution and good shape-uniformity confirmed by TEM image (Fig. 2b). The high-resolution TEM image showed obvious crystal lattice fringes. Fig. 2c showed the XRD patterns of the QDs, the strong broad peaks around 2θ values of 24.2°, 26.6°, 28.3°, 44.5° and 48.1° attributed to (120)/(200), (002), (121)/(201), (040)/(320) and (123) phases of orthorhombic AgInS2 (JCPDS No.25-1328). The broadening diffraction peaks suggested the small sizes of QDs. EDX measurement in Fig. 2d confirmed the presence of Ag, In and S elements with an atomic ratio of 1:1.2:1.9, which was close to stoichiometry of AgInS2. From the FT-IR spectra (Fig. 2e), the bands 1570 cm-1 and 1396 cm-1 could be assigned to the C-O symmetric and asymmetric vibration of COO- group and the bands 2923 cm-1 and 2852 cm-1 referred to the asymmetric and symmetric stretching vibrations of C-H of MPA. The band around 1273 cm-1 attributed to the bending vibrations of CH2-S and the absence of a peak at 2490 cm-1 suggested the inexistence of free SH-, which demonstrated that MPA molecules were bound to the surface of AgInS2 QDs as ligands. The XPS result also illustrated that the valence states of the ions were Ag+, In3+ and S2-, certifying the formation of AgInS2 in the droplet microreactors (Fig. S4 in Supporting information). The AgInS2 QDs achieved QYs of 0.8%-8.8% with Rhodamine 101 as a reference standard (Table S1 in Supporting information). The maximum QY could reach 8.8% when the reaction temperature was 70 ℃.
|
Download:
|
| Fig. 2. (a) Offline fluorescence and absorption spectra of AgInS2 QDs synthesized in droplets at 50 ℃, UW = 20 mL/h, UO = 40 mL/h. (b) TEM image of QDs. Inset: a highresolution TEM image. (c–e) XRD, EDX, FT-IR spectra. (f) The picture of a series of AgInS2 fluorescent QDs synthesized at different reaction temperature (from left to right: 30–70 ℃). | |
Temperature often plays a key role during colloidal QDs synthesis. In this work, the effect of temperature on AgInS2 QDs fluorescence properties was studied by changing the reaction temperature from 30 ℃ to 70 ℃, while all other experimental conditions remained the same. Figs. 3a and b show the UV-vis absorption spectra and normalized fluorescence spectra of the products at different temperatures. There were no obvious absorption peaks, which might be ascribed to the wide size distribution or trap-state-related emission [25]. Meanwhile, reaction temperature also influenced the fluorescence intensity of QDs. When the reaction was operated below 30 ℃, no significant fluorescence emission was found (data not shown), indicating that the synthesis of AgInS2 QDs could not be realized at such low temperature. When the reaction temperature increased from 30 ℃ to 70 ℃, the fluorescence peak of the QDs constantly red-shifted from 590 nm to 720 nm and the QY was also enhanced (Table S1), therefore, the fluorescence intensity was improved (Fig. 3c). TEM images in Figs. 3d-f also showed that the particle size increased from 3.5 nm to 4.4 nm as the temperature increased (30, 50, 70 ℃). However, when synthesized at a higher temperature (80 ℃), QY decreased to 5.8%, the fluorescence intensity of the QDs decreased obviously. We speculated that when reaction temperature was low, the system could not provide enough energy for ordered atom rearrangement on the surface of the QDs, leading to more surface defects and low fluorescence QY and intensity. As the reaction temperature increased, the surface defects decreased and the fluorescence intensity increased. But when the reaction temperature was too high (80 ℃), the growth rate of nanocrystals became too fast to control, resulting in fluorescence QY and intensity decreased [6, 26]. Therefore, appropriate reaction temperature could lead to higher QYand higher fluorescence intensity of AgInS2 QDs.

|
Download:
|
| Fig. 3. (a), (b) Absorption spectra and online fluorescence spectra of AgInS2 QDs synthesized in droplets at different temperatures from 30 ℃ to 70 ℃. (c) Plot of AgInS2 QDs fluorescence intensity upon reaction temperature. (d-f) TEM images and corresponding size distribution histograms. | |
Furthermore, we continuously recorded the fluorescence spectra of QDs products within the droplets at a fixed position of the chip for 5 h. Fig. 4a shows that there was no obvious change, indicating good stability of the droplet microreactors. Besides, five chips were used for synthesis under the same conditions, and the fluorescence spectra of the products was nearly the same, showing good reproducibility of the droplet microreactors (Fig. 4b).

|
Download:
|
| Fig. 4. (a) Time evolution of online fluorescence spectra of AgInS2 QDs synthesized in droplets. Reaction conditions were fixed at 50 ℃, UW = 20 μL/h, UO = 40 μL/h. (b) Online fluorescence spectra of AgInS2 QDs synthesized in different batch chips keeping reaction conditions fixed at 52 ℃, UW = 20 μL/h, UO = 40 μL/h. | |
In conclusion, the temperature-controllable droplet microreactor with good stability and reproducibility was designed to synthesize the water-soluble AgInS2 QDs, and the temperature influence on the fluorescence properties of AgInS2 QDs was studied. Results indicated that the monodispersed orthorhombic AgInS2 QDs with MPA as surface ligands were successfully synthesized in droplet microreactors, and the reaction time was shorter than that in the flask. When reaction temperature increased from 30 ℃ to 70 ℃, QDs size ranged from 3.5 nm to 4.4 nm, and the fluorescence peak constantly red-shifted from 590 nm to 720 nm along with enhanced fluorescence QY and intensity. AgInS2 QDs with the maximum fluorescence intensity and the QY of 8.8% could be obtained at 70 ℃.
In addition, droplet microreactors showed unique advantages in the synthesis of AgInS2 QDs which were simple and able to precisely change and control the reaction conditions. Moreover, for the ternary QDs, droplet microreactors might better regulate the reactant composition and reaction conditions, thus effectively balancing the reactivity between the two cations, and synthesizing ternary QDs with less defects and higher fluorescence efficiency.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21375100, 21775111) and the National Science and Technology Major Project of China (No. 2018ZX10301405).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.04.033
| [1] |
H.Z. Zhong, Z.L. Bai, B.S. Zou, J. Phys. Chem. Lett. 3 (2012) 3167-3175. DOI:10.1021/jz301345x |
| [2] |
T. Pons, E. Pic, N. Lequeux, et al., ACS Nano 4 (2010) 2531-2538. DOI:10.1021/nn901421v |
| [3] |
P.M. Allen, M.G. Bawendi, J. Am. Chem. Soc. 130 (2008) 9240-9241. DOI:10.1021/ja8036349 |
| [4] |
R.G. Xie, M. Rutherford, X.G. Peng, J. Am. Chem. Soc. 131 (2009) 5691-5697. DOI:10.1021/ja9005767 |
| [5] |
G. Manna, S. Jana, R. Bose, N. Pradhan, J. Phys. Chem. Lett. 3 (2012) 2528-2534. |
| [6] |
W.M. Girma, M.Z. Fahmi, A. Permadi, M.A. Abate, J.Y. Chang, J. Mater. Chem. B 5 (2017) 6193-6216. |
| [7] |
L.W. Liu, R. Hu, W.C. Law, et al., Analyst 138 (2013) 6144-6153. DOI:10.1039/c3an01030a |
| [8] |
T. Ogawa, T. Kuzuya, Y. Hamanaka, K. Sumiyama, J. Mater. Chem. 20 (2010) 2226-2231. DOI:10.1039/b920732e |
| [9] |
T. Torimoto, S. Ogawa, T. Adachi, et al., Chem. Commun. 46 (2010) 2082-2084. DOI:10.1039/b924186h |
| [10] |
M. Dai, S. Ogawa, T. Kameyama, et al., J. Mater. Chem. 22 (2012) 12851-12858. DOI:10.1039/c2jm31463k |
| [11] |
M.Z. Fahmi, J.Y. Chang, Nanoscale 5 (2013) 1517-1528. DOI:10.1039/c2nr33429a |
| [12] |
Z.S. Luo, H. Zhang, J. Huang, X.H. Zhong, J. Colloid Interf. Sci. 377 (2012) 27-33. DOI:10.1016/j.jcis.2012.03.074 |
| [13] |
W.W. Xiong, G.H. Yang, X.C. Wu, J.J. Zhu, J. Mater. Chem. B 1 (2013) 4160-4165. DOI:10.1039/c3tb20638f |
| [14] |
K. Kim, R.P. Oleksak, E.B. Hostetler, et al., Cryst. Growth Des. 14 (2014) 5349-5355. DOI:10.1021/cg500959m |
| [15] |
A. Yashina, I. Lignos, S. Stavrakis, J. Choo, A.J. deMello, J. Mater. Chem. C 4 (2016) 6401-6408. DOI:10.1039/C6TC02057G |
| [16] |
L.J. Pan, J.W. Tu, H.T. Ma, et al., Lab Chip 18 (2018) 41-56. DOI:10.1039/C7LC00800G |
| [17] |
C.G. Yang, R.Y. Pan, Z.R. Xu, Chin. Chem. Lett. 26 (2015) 1450-1454. DOI:10.1016/j.cclet.2015.10.016 |
| [18] |
K.S. Elvira, X.C. Solvas, R.C.R. Wootton, A.J. deMello, Nat. Chem. 5 (2013) 905-915. DOI:10.1038/nchem.1753 |
| [19] |
H. Song, J.D. Tice, R.F. Ismagilov, Angew. Chem. Int. Ed. 42 (2003) 768-772. DOI:10.1002/anie.200390203 |
| [20] |
Y. Shu, P. Jiang, D.W. Pang, Z.L. Zhang, Nanotechnology 26 (2015) 275701. DOI:10.1088/0957-4484/26/27/275701 |
| [21] |
M.S. Jie, S.F. Mao, H.F. Li, J.M. Lin, Chin. Chem. Lett. 28 (2017) 1625-1630. DOI:10.1016/j.cclet.2017.05.024 |
| [22] |
I. Lignos, S. Stavrakis, G. Nedelcu, et al., Nano Lett. 16 (2016) 1869-1877. DOI:10.1021/acs.nanolett.5b04981 |
| [23] |
S. Yao, Y. Shu, Y.J. Yang, et al., Chem. Commun. 49 (2013) 7114-7116. DOI:10.1039/c3cc42503g |
| [24] |
I. Tsuji, H. Kato, H. Kobayashi, A. Kudo, J. Am. Chem. Soc. 126 (2004) 13406-13413. DOI:10.1021/ja048296m |
| [25] |
W.J. Zhang, X.H. Zhong, Inorg. Chem. 50 (2011) 4065-4072. DOI:10.1021/ic102559e |
| [26] |
D.C. Che, X.X. Zhu, H.Z. Wang, et al., J. Colloid Interf. Sci. 463 (2016) 1-7. DOI:10.1016/j.jcis.2015.10.039 |
 2019, Vol. 30
2019, Vol. 30 

