b The Education Ministry Key Laboratory of Resource Chemistry and Shanghai Key Laboratory of Rare Earth Functional Materials, Department of Chemistry, College of Life and Environmental Sciences, Shanghai Normal University, Shanghai 200234, China;
c Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, Shanghai 200237, China
Zinc, like calcium and iron, as an essential transition metal in the human body, takes part in many important process of life. Zinc is not only catalytic center and structural cofactor of many biomacromolecules, such as enzymes and DNA-binding proteins, but also a signaling messenger released by many central excitatory synapses, it also plays an important role in central nervous system [1]. It has been revealed that the origins and consequences of zinc signalsare closely related with the incidence of certain diseases, like prostate cancer [2], Alzheimer's disease [3], epilepsy [4].
Lysosome degradation, as the final process of autophagy, is also important in the process of protein degradation [5], and the degradation process would producewaste protein and other waste in cells. In general, it works slowly under normal conditions, but can be influenced by oxidative stress [6]. Disordered lysosome degradation will hurt normal proteins and organelles, and might be involved in part of the pathogenesis of many cancers and neurodegenerative diseases [7-9]. In recent years, it has been investigated that zinc ions may be related with lysosome membrane permeabilization (LMP) in oxidative process [10], and LMP plays important roles in oxidative and zinc-induced hippocampal neuronal death. In order to explore the relationship between zinc and LMP, we need an efficient approach to further study zinc ions in lysosome. Because of Zinc's 3d104s0 electron configuration, many of the existing methods are not suitableforthe detection of zinc in cells [11], like UV spectrum, circular dichroism spectrum, electron paramagnetic resonance, cyclic voltammetry and atomic absorption spectrum (AAS).
Fluorescent probe has been provided as an efficiency approach to detect metal ions in the past decades [12-17]. Roger Tsien first reported a series of probes for calcium ions, which could imaging Ca2+ in living cells [18]. From then on, many fluorescent chemical probes and biosensors have been developed for different metal ions. Frederickson designed the first fluorescent probe TSQ for Zn2+, based on an aryl sulfonamide at 1987 [19]. After that, based on different fluorophores, a large number of fluorescent probes have been developed for sensing zinc ions. As a typical Zn2+ receptor, di-2-picolylamine (DPA) has been widely used to construct Zn2+ probe. For example, Lippard et al. reported probes ZPs and ZSs to detect zinc ions in different living cells, organelles or hippocampal mossy fiber [20-25]. Wang et al. developed a highly sensitive zinc probe WZS with the apparent dissociation constant Kd of 0.62 nmol/L [26]. Xu et al. reported a highly selective sensor ZTRS for ratiometric Zn2+ sensing, which is cell permeable and can be applied to trace zinc ions during the development of a living organism [27]. Moreover, Yoon et al. had designed a zinc probe based on naphthalimide fluorophore, which could image Zn2+ concentration changes in the vicinity of NMDA receptors in live cells and tissues [28]. Based on these zinc probes, more and more evidences showed that intracellular zinc ions are related with lysosome, H2O2 and other active materials [29, 30]. Although some probes have been developed to detect Zn2+ in lysosome [31-33] to explore the significance of Zn2+ in lysosomal membrane permeabilization, few of them were reported to study the relationship between zinc ions and oxidative stress in neuroblastoma cells.
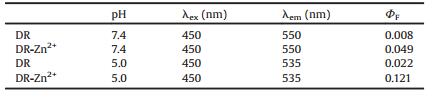
Since a protonated morpholine has a pKa value of ~5, a lysosomal pH, and N, N-bis(2-pyridylmethyl)ethylenediamine (BPEN) is a good receptor for Zn(Ⅱ). Herein, we developed a fluorescent probe DR, which bearing a morpholine group and a BPEN ligand. By attaching BPEN to the fluorophore through a rigid benzene framework on the imide moiety, DR exhibited high sensitivity to Zn(Ⅱ). And the morpholine moiety in the 4-position of naphthalimide fluorophore enabled the lysosomal targeting of the probe (Scheme 1)
|
Download:
|
| Scheme 1. Response mechanism of fluorescent probe DR. | |
Probe DR can be readily synthesized from 4-nitro-1, 8-naphthalic anhydride as we previously reported (Scheme S1 in Supporting information) [34]. Based on the fluorescent recognition mechanism of photo-induced electron transfer (PET), BPEN was introduced into the imide moiety of the anphthalimide fluorophore as the receptor of Zn2+, N-(2-aminoethyl)morpholine moiety was introduced into the 4-position of naphthalimide as the receptor of hydrogen proton. The introduction of morpholine not only enables the lysosomal targeting of the probe, but also improves the aqueous solubility of the probe. The detailed synthesis procedures of the probe DR were present in Supporting information.
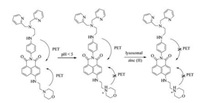
We first conducted the pH-titration of the probe DR and its Zn(Ⅱ) complex. The pH value of the test system was adjusted by HCl and NaOH solution. As shown in Fig. 1A and Table 1, probe DR emits very weak fluorescence at pH 7.0-10.0 with a fluorescence quantum yield of 0.009 (pH 7.0), which can be attributed to the PET processes both from the aniline nitrogen and the morpholine nitrogen atom. And 2-butyl-6-(butylamino)-1H-benzo[de]isoquinoline-1, 3(2H)-dione with a fluorescence quantum yield of 0.66 in ethanol was used as the standard reference in this work [35]. With the decrease of the pH value from 7.0 to 4.0, the fluorescence intensity of the probe DR had a little enhancement (ΦF = 0.022, pH 5.0). This can be explained by that the nitrogen-atom of morpholine moiety was protonated gradually, which inhibited the PET process from the nitrogen-atom of morpholine to fluorophore. When DR combined with Zn2+, the fluorescence intensity enhanced at pH 7.0-10.0 (ΦF = 0.049, pH 7.4). While in the range of pH 7.5-4.0, the fluorescence intensity increased remarkably, with 13.4 folds increase of the fluorescence quantum yield from 0.008 (probe DR, pH 7.4) to 0.121 (DR-Zn2+, pH 5.0), which can be attributed to the suppressed PET processes both from the Zn(Ⅱ)- complexed BPEN site as well as from the protonated nitrogen of morpholine moiety. These results suggested that the probe DR had potential to stain lysosome in cells and sense free zinc in lysosome.
|
Download:
|
| Fig. 1. (A) Changes of fluorescent intensity of DR (black) and DR-Zn2+ (red) under different pH (adjusted by 1 mol/L HCl or 1 mol/L NaOH) in water (0.5% DMSO) solution. (B) Fluorescence spectra of DR upon addition of Zn2+. Inset: Changes of intensity plots of DR vs. different concentrations of Zn2+. Conditions: 0.1 mol/L TrisHCl buffer (0.5% DMSO, pH 5.0), [DR] = 5 μmol/L, λex = 450 nm, λem = 550 nm, 25 ℃. | |
|
|
Table 1 Maximum of adsorption, emission and fluorescence quantum yield of DR and DR-Zn2+ at different pH. |
In order to investigate the probe DR's potential for sensing lysosomal zinc, we further performed the Zn(Ⅱ) titration experiments at pH 5.0. As shown in Fig. 1B, upon addition of Zn2+ to the probe's solution (0-10 μmol/L, 0.1 mol/L Tris-HCl, 0.5% DMSO, pH 5), the fluorescence emission intensity of DR increased gradually with the increase of the Zn(Ⅱ) concentration, and reached a plateau finally. The fluorescence intensity showed a linear enhancement (correlation coefficient R2 = 0.998, Fig. S1 in Supporting information) before the ratio of Zn(Ⅱ):DR reached 1:1, and the Zn(Ⅱ)- binding titrations showed that DR forms a 1:1 complex with Zn(Ⅱ) in aqueous solution (Fig. S2 in Supporting information). The association constant was calculated to be 4.9 × 108 L/mol, and the detection limit was 15 nmol/L based on LOD = 3σ/s. These results revealed that the probe DR could sense trace lysosomal Zn2+ in living cells.
Next, we explored the specificity of DR (Fig. S3 in Supporting information). In the presence of Na+, K+, Ca2+ and Mg2+, no fluorescence enhancement was observed, while the fluorescence increased remarkably with the further addition of Zn(Ⅱ). Cu(Ⅱ) quenched the fluorescence of DR owing to the paramagnetic effect. Similar to other Zn2+ probes containing DPA units, Cd2+, Hg2+, Pb2+ also induced fluorescence enhancement, but less than Zn2+. Since there were few Cd2+, Hg2+, Pb2+ in normal human cells, the probe DR should have the potential in imaging intracellular zinc ions.
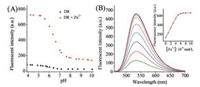
Then we investigated the locating capability of DR in acidic vesicle of living cells by performing a co-localization experiment with a commercial marker for lysosomes (LysoTracker® Red) in live MCF-7 cells. MCF-7 cells were stained with LysoTracker® Red and DR and co-incubated for 1 h, then imaged with confocal microscopy. As shown in Fig. 2, the images of green channel (probe DR) and red channel (LysoTracker® Red) overlapped very well, suggesting that DR could be used as a lysosome-tracker probe in living cells.
|
Download:
|
| Fig. 2. Confocal fluorescence images of MCF-7 cells: (A) Stained with DR (5 μmol/L, λex = 488 nm, λem = 520–570 nm), (B) stained with LysoTracker Red (50 nmol/L, λex = 561 nm, λem = 570–675 nm), (C) merge of (A) and (B), (D) the bright field image. All the cells were magnified 1560 times. Scale bar: 15 μm. | |
It is known that zinc(Ⅱ) is related with oxidative stress in living systems. For example, when Zn(Ⅱ)-bound cysteine residues are oxidized to form disulfide bonds, Zn2+ ions are released to result in an increase of local Zn(Ⅱ) concentrations. Such oxidation-effected Zn(Ⅱ) release may be linked to the formation of protein aggregates, which are significant in neurodegenerative diseases such as Alzheimer's and Parkinson's [36]. Hence, we finally used the probe DR to investigate the effect of oxidative stress in SH-SY5Y cells. In this experiment, we treated SH-SY5Y cells in three different ways: (A) SH-SY5Y cells were stained with DR; (B) SH-SY5Y cells were coincubation with H2O2 for 1 h, then stained with DR; (C) SH-SY5Y cells were co-incubation with H2O2 for 1 h, followed by the additon of TPEN (one kind of chelating agent for zinc) and stained with DR. As shown in Fig. 3, after SH-SY5Y cells were treated with H2O2, the green fluorescence enhanced significantly (Fig. 3B) compared with those non-treated cells (Fig. 3A), which revealed the release of Zn2+ after stimulating by H2O2. When cells were simultaneously treated with H2O2 and TPEN, the fluorescence got very dark (Fig. 3C), which further indicated that such fluorescence changes were related to the changes of Zn(Ⅱ) concentrations in SH-SY5Y cells. These results demonstrate that DR has the potential in imaging free zinc in human neuroblastoma cells.
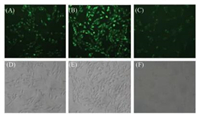
|
Download:
|
| Fig. 3. Fluorescence images of DR (5 μmol/L) in (A) SH-SY5Y cells, (B) SH-SY5Y cells cultured with H2O2, (C) SH-SY5Y cells cultured with H2O2 and 10 μmol/L TPEN, (D, E, F) bright field images of (A), (B) and (C), respectively. The excitation light was blue light and cells were magnified 200 times. | |
In this work, we had designed a fluorescent sensor DR based on BPEN ligand and the target group morpholine. DR had a low fluorescence quantum yield (ΦF = 0.008) owing to the electron transfer from the aniline nitrogen and the morpholine nitrogen to fluorophore at pH ~7.4. Upon binding Zn2+, the fluorescence quantum yield of DR enhanced about 13.4 folds at pH ~5.0, which exhibited high sensitivity to Zn2+ and the detection limit of was calculated to be 15 nmol/L. Confocal imaging experiments indicated that DR was able to localize to lysosomes in MCF-7 cells. Moreover, upon H2O2 stimulation in SH-SY5Y cells, endogenous release of Zn2+ was observed, which was demonstrated by the fluorescence changes after the cells were treated with H2O2 and the membrane-permeable zinc chelator TPEN.
AcknowledgmentsWe thank the National Natural Science Foundation of China (No. 21476077) for financial support. We also very much appreciate Prof. Xuhong Qian's valuable suggestions in the preparation of this manuscript.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.03.016.
| [1] |
N. Roohani, R. Hurrell, R. Kelishadi, R. Schulin, J. Res. Med. Sci. 18 (2013) 144-157. |
| [2] |
R.B. Franklin, L.C. Costello, Arch. Biochem. Biophys. 463 (2007) 211-217. DOI:10.1016/j.abb.2007.02.033 |
| [3] |
O. Timofeeva, J.V. Nadler, Brain Res. 1078 (2006) 227-234. DOI:10.1016/j.brainres.2006.01.051 |
| [4] |
K. Golovine, R.G. Uzzo, P. Makhov, et al., Prostate 68 (2008) 1443-1449. DOI:10.1002/pros.v68:13 |
| [5] |
G. Kroemer, M. Jäättelä, Nat. Rev. Cancer 5 (2005) 886-897. DOI:10.1038/nrc1738 |
| [6] |
M. Kundu, C.B. Thompson, Annu. Rev. Pathol. Mech. Dis. 3 (2008) 427-455. DOI:10.1146/annurev.pathmechdis.2.010506.091842 |
| [7] |
Y. Uchiyama, M. Koike, M. Shibata, M. Sasaki, Methods Enzymol. 453 (2009) 33-51. DOI:10.1016/S0076-6879(08)04003-2 |
| [8] |
B. Levine, Nature 446 (2007) 745-747. DOI:10.1038/446745a |
| [9] |
B. Levine, G. Kroemer, Cell 132 (2008) 27-42. DOI:10.1016/j.cell.2007.12.018 |
| [10] |
J.J. Hwang, S.J. Lee, T.Y. Kim, et al., J. Neurosci. 28 (2008) 3114-3122. DOI:10.1523/JNEUROSCI.0199-08.2008 |
| [11] |
S.Y. Assaf, S.H. Chung, Nature 308 (1984) 734-736. DOI:10.1038/308734a0 |
| [12] |
K.P. Carter, A.M. Young, A.E. Palmer, Chem. Rev. 114 (2014) 4564-4601. DOI:10.1021/cr400546e |
| [13] |
Z. Xu, J. Tang, H. Tian, Chin. Chem. Lett. 19 (2008) 1353-1357. DOI:10.1016/j.cclet.2008.09.005 |
| [14] |
H.Y. Zhao, Y.M. Li, T.J. Gong, Q.X. Guo, Chin. Chem. Lett. 22 (2011) 1013-1016. DOI:10.1016/j.cclet.2011.03.007 |
| [15] |
C.X. Ding, C.H. He, Y.S. Xie, Chin. Chem. Lett. 24 (2013) 463-466. DOI:10.1016/j.cclet.2013.03.030 |
| [16] |
W. Feng, Q.L. Qiao, S. Leng, et al., Chin. Chem. Lett. 27 (2016) 1554-1558. DOI:10.1016/j.cclet.2016.06.016 |
| [17] |
M.N. Su, J. Ye, Q.W. Li, et al., Chin. Chem. Lett. 26 (2015) 1400-1402. DOI:10.1016/j.cclet.2015.07.021 |
| [18] |
R.Y. Tsien, Biochemistry 19 (1980) 2396-2404. DOI:10.1021/bi00552a018 |
| [19] |
C.J. Frederickson, E.J. Kasarskis, D. Ringo, R.E. Frederickson, J. Neurosci. Methods 20 (1987) 91-103. DOI:10.1016/0165-0270(87)90042-2 |
| [20] |
S.C. Burdette, G.K. Walkup, B. Spingler, R.Y. Tsien, S.J. Lippard, J. Am. Chem. Soc. 123 (2001) 7831-7841. DOI:10.1021/ja010059l |
| [21] |
X.A. Zhang, D. Hayes, S.J. Smith, S. Friedle, S.J. Lippard, J. Am. Chem. Soc. 130 (2008) 15788-15789. DOI:10.1021/ja807156b |
| [22] |
C.C. Woodroofe, S.J. Lippard, J. Am. Chem. Soc. 125 (2003) 11458-11459. DOI:10.1021/ja0364930 |
| [23] |
B.A. Wong, S. Friedle, S.J. Lippard, J. Am. Chem. Soc. 131 (2009) 7142-7152. DOI:10.1021/ja900980u |
| [24] |
R.J. Radford, W. Chyan, S.J. Lippard, Chem. Sci. 4 (2013) 3080-3084. DOI:10.1039/c3sc50974e |
| [25] |
M. Khan, C.R. Goldsmith, H. Zhen, et al., Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 6786-6791. DOI:10.1073/pnas.1405154111 |
| [26] |
J. Wang, Y. Xiao, Z. Zhang, et al., J. Mater. Chem. 15 (2005) 2836. DOI:10.1039/b500766f |
| [27] |
Z. Xu, K.H. Baek, H.N. Kim, et al., J. Am. Chem. Soc. 132 (2010) 601-610. DOI:10.1021/ja907334j |
| [28] |
X. Chen, C.S. Lim, D. Lee, et al., Biosens. Bioelectron. 91 (2017) 770-779. DOI:10.1016/j.bios.2017.01.042 |
| [29] |
S.J. Lee, J.Y. Koh, Mol. Brain 3 (2010) 30-38. DOI:10.1186/1756-6606-3-30 |
| [30] |
L.M. Hyman, K.J. Franz, Coord. Chem. Rev. 256 (2012) 2333-2356. DOI:10.1016/j.ccr.2012.03.009 |
| [31] |
H. Zhu, J. Fan, S. Zhang, et al., Biomater. Sci. 2 (2014) 89-97. DOI:10.1039/C3BM60186B |
| [32] |
S.L. Kelleher, N.H. McCormick, V. Velasquez, V. Lopez, Adv. Nutr. 2 (2011) 101-111. DOI:10.3945/an.110.000232 |
| [33] |
L. Xue, G. Li, D. Zhu, Q. Liu, H. Jiang, Inorg. Chem. 51 (2012) 10842-10849. DOI:10.1021/ic301307v |
| [34] |
X. Qian, Y. Xiao, Y. Xu, et al., Chem. Commun. 46 (2010) 6418-6436. DOI:10.1039/c0cc00686f |
| [35] |
D. Yuan, R.G. Brown, J.D. Hepworth, M.S. Alexiou, J.H.P. Tyman, J. Heterocycl. Chem. 45 (2008) 397-404. DOI:10.1002/jhet.v45:2 |
| [36] |
L. Zhu, Z. Yuan, J.T. Simmons, K. Sreenath, RSC Adv. 4 (2014) 20398-20440. DOI:10.1039/C4RA00354C |
 2019, Vol. 30
2019, Vol. 30