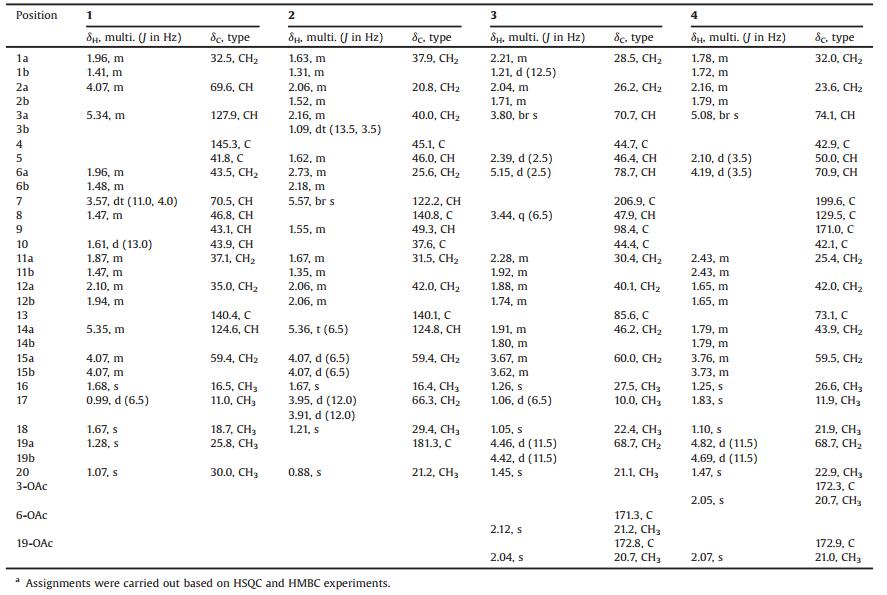
Leonurus macranthus Maxim., family Lamiaceae (syn. Labiatae), is a perennial herbaceous plant mainly distributed in northeast Asia. The dried aerial parts of L. macranthus are used as motherwort locally for the treatment of menoxenia, dysmenorrhea, amenorrhea, lochia, edema, oliguresis, and ulcerations [1, 2]. Our previously phytochemical investigations of this plant revealed the presence of diterpenoids, lignans, flavonoids, coumarin, and phenolic acids [2, 3]. As a continued program to search for bioactive diterpenoids from medicinal plants [2, 4, 5], four new diterpenoids including one cis clerodane-type (1) and three highly oxygenated labdane-type diterpenes (2-4) (Fig. 1) were isolated from the acetone extract of the aerial parts of L. macranthus. Herein, the isolation and structural elucidation of the new compounds are described as well as their inhibitory effects on nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated BV-2 microglial cells.
|
Download:
|
| Fig. 1. Structures of compounds 1-4 from Leonurus macranthus. | |
The acetone extract of the aerial parts of L. macranthus was processed by solvent partitioning with n-hexane and CH2Cl2, successively. The CH2Cl2-soluble fraction was repeatedly subjected to silica gel, Sephadex LH-20, and Lichroprep RP-C18 gel column chromatography (CC) and semipreparative RP-C18 HPLC to afford one new cis clerodane-type (1) and three new labdane-type diterpenes (2-4). Detailed experimental procedures and full spectroscopic data for compounds 1-4 can be found in Supporting information.
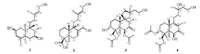
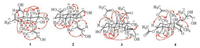
Macranthin H (1) was obtained as a colorless gum, [α]D21 -30. Its 13C NMR and negative-ion HRESIMS data, exhibiting a deprotonated molecular ion peak at m/z 321.2425, corresponded to a molecular formula of C20H34O3 (calcd. for C20H33O3: 321.2435), with four indices of hydrogen deficiency. The IR absorption band at 3419 cm-1 was indicative of the presence of hydroxy group. The 1H NMR data (Table 1) displayed characteristic resonances of five methyls [δH 1.07, 1.28, 1.67, and 1.68 (s, each 3H); δH 0.99 (d, 3H, J = 6.5 Hz)], one oxygenated methylene [δH 4.07 (2H, m)], two oxygenated methines [δH 3.57 (dt, 1H, J = 11.0, 4.0 Hz); δH 4.07 (m, 1H)], and two olefinic protons [δH 5.34 (m, 1H); δH 5.35 (m, 1H)]. The 13C NMR data (Table 1) showed 20 carbon resonances comprising five methyls, five sp3 methylenes, four sp3 methines, two sp3 quaternary carbons, and four sp2 carbons. These functionalities accounted for two out of the four indices of hydrogen deficiency, requiring the presence of two rings in 1. The aforementioned data suggested that 1 is a clerodane diterpenoid [8]. The 1H NMR and 13C NMR spectroscopic data (Table 1) of 1 were comparable to those of kolavenol [8], which was previously isolated from the root bark of Entada abyssinica. The significantly deshielded C-2 (δC 69.6) and C-7 (δC 70.5) resonances as well as the HMBC correlations from H2-1/H-10 to C-2, from H-2 to C-3/C-4, from H2-6/H-8/H3-17 to C-7, and from H-7 to C-17 (Fig. 2) indicated that C-2 and C-7 are oxygenated. The planar structure of 1 was further supported by HMBC correlations from H-3 to C-5/C-18, from H2-12 to C-11/C-14, from H2-15 to C-13/C-14, from H3-16 to C-12/C-13/C-14, from H3-18 to C-3/C-5, H3-19 to C-4/C-6/C-10, and from H3-20 to C-8/C-10/C-11, as well as 1H-1H COSY correlations of H-10/H2-1, H2-1/H-2, H-2/H-3, H2-6/H-7, H-7/H-8, and H-8/H3-17 (Fig. 2). In the NOESY spectrum (Fig. 3), the NOE correlation between H3-19 and H-10 supported the cis-fusion of A/B rings in 1. The NOE correlations of H-7/H2-11, H-7/H3-17, H-7/H3-19, H-10/H2-11, H-10/H3-19, H2-11/H3-19, and H3-19/6α indicated that these hydrogens are cofacial and α-oriented, while NOEs of H-8/H3-20, H-8/H-6β, and H-1β/H-6β suggested that these protons are β-cofacially oriented. The presence of NOE correlation of H-2/H-10 supported 2-OH to be β-oriented. NOEs of H2-15/H3-16, H2-12/H-14 indicated E configuration of the double bond between C-13 and C-14. Therefore, the structure of 1 (macranthin H) was characterized as 2β, 7β-dihydroxy-(5α, 8α, 9β, 10α)-cleroda-3, 13E-dien-15-ol.
|
|
Table 1 1H NMR (500 MHz) and 13C NMR (125 MHz) data of compounds 1-4 (δ in ppm, in methanol-d4).a |
|
Download:
|
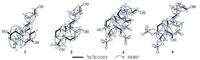
| Fig. 2. 1H-1H COSY and selected HMBC (arrows point from protons to carbons) correlations of compounds 1-4. | |
|
Download:
|
| Fig. 3. Selected NOE correlations of compounds 1-4. | |
It is worth to note that the cis-fusion of rings A/B is rarely encountered in clerodane-type diterpenoids [9]. Compound 1 represents the first example of cis clerodane-type diterpene in the plants of Leonurus genus.
Macranthin I (2) gave a molecular formula of C20H32O4 based on the 13C NMR data and a deprotonated ion peak at m/z 335.2235 [M-H]- (calcd. for C20H31O4: 335.2228) in the negative-ion HRESIMS. The IR spectrum indicated the presence of hydroxy (3432 cm-1) and carboxyl carbonyl (1693 cm-1) functionalities. The 1H NMR data (Table 1) displayed resonances of three methyls [δH 0.88, 1.21, 1.67 (s, each 3H)], two oxygenated methylenes [δH 3.95 (d, 1H, J = 12.0 Hz), 3.91 (d, 1H, J = 12.0 Hz); δH 4.07 (d, 2H, J = 6.5 Hz)], and two olefinic protons [δH 5.36 (t, 1H, J = 6.5 Hz); δH 5.57 (br s, 1H)]. The 13C NMR data (Table 1) of 2 showed 20 carbon resonances due to three methyls, eight methylenes (two oxygenated), four methines (two olefinic), and five quaternary carbons (one carbonyl, two olefinic). Detailed analysis of the 1H NMR and 13C NMR spectroscopic data (Table 1) of 2showed a close structural resemblance to leojaponin B [10], which was previously isolated from Leonurus japonicus. The major difference was the replacement of the C-17 methyl group in leojaponin B by a hydroxymethyl group in 2. This deduction was supported by the significantly deshielded H2-17 [δH 3.95 (d, 1H, J = 12.0 Hz), 3.91 (d, 1H, J = 12.0 Hz); ΔδH +2.31, 2.27] and C-17 (δC 66.3; ΔδC+43.0) resonances as well as the HMBC correlations from H2-17 to C-7/C-8/C-9 (Fig. 2). The 2D structure of 2 was also supported by 1H-1H COSY correlations of H2-1/H2-2, H2-2/H2-3, H-5/H2-6, H2-6/H-7, H-9/H2-11, H2-11/H2-12, and H-14/H2-15 (Fig. 2). The relative configuration of 2 was established on the basis of NOESYdata (Fig. 3). The NOE correlations of H3-20/H-6β and H-5/H-6α supported the trans A/B junction in 2. NOEs of H-5/H-6α, H-5/H2-11, and H-5/H3-18 indicated that these protons are on the same face and α-oriented, while the correlations of H3-20/H-6β and H3-20/H-9 suggested that these protons are β-oriented. The NOE correlations of H2-15/H3-16, H2-12/H-14 indicated that the double bond between C-13 and C-14 is E configuration. Thus, the structure of 2 (macranthin I) was elucidated as 15, 17-dihydroxylabd-7, 13E-dien-19-oic acid.
Compound 3 was obtained as colorless gum, [α]D21-30. Its molecular formula, C24H38O8, was deduced from the HRESIMS (m/z 455.2645 [M + H]+, calcd. for C24H39O8: 455.2639) and 13C NMR spectroscopic data, indicating six indices of hydrogen deficiency. The IR spectrum showed absorption bands for hydroxy (3545 cm-1) and carbonyl (1731 cm-1) functionalities. The 1H NMR data (Table 1) displayed characteristic resonances for three tertiary methyl [δH 1.05, 1.26, and 1.45 (s, each 3H)], one secondary methyl [δH 1.06 (d, 3H, J = 6.5 Hz)], two oxygenated methylene [δH 3.62 (m, 1H), 3.67 (m, 1H); δH 4.42 (d, 1H, J = 11.5 Hz), 4.46 (d, 1H, J = 11.5 Hz)], two oxygenated methine [δH 3.80 (br s, 1H); δH 5.15 (d, 1H, J = 2.5 Hz)], and two O-acetyl [δH 2.04, 2.12 (s, each 3H)] groups. The 13C NMR data (Table 1) of 3 showed 24 carbon resonances due to six methyl (δC 10.0, 20.7, 21.1, 21.2, 22.4, 27.5), seven sp3 methylene (δC 26.2, 28.5, 30.4, 40.1, 46.2, 60.0, 68.7), four sp3 methine (δC 46.4, 47.9, 70.7, 78.7), four quaternary sp3 (δC 44.4, 44.7, 85.6, 98.4), two ester carbonyl (δC 171.3 and 172.8), and one keto carbonyl (δC 206.9) carbons. These functionalities accounted for three out of the six indices of hydrogen deficiency, requiring the presence of a tricyclic system in 3. The aforementioned data suggested that 3 is a highly oxygenated tricyclic spirolabdane diterpenoid [2, 11]. The 1H NMR and 13C NMR spectroscopic data (Table 1) of 3 were comparable to those of macranthin A [2], except for the absence of resonances of an acetoxy group in 3. The deshielded resonances of H-6 (δH 5.15) and H2-19 (δH 4.42, 4.46) indicated that two O-acetyl groups are located at C-6 and C-19, respectively. This was confirmed by the HMBC correlations from H-6 and H2-19 to the acetoxy carbonyl carbons at δC 171.3 and 172.8, respectively (Fig. 2). The planar structure of 3 was further supported by 1H-1H COSY correlations of H2-1/H2-2, H2-2/H-3, H-5/H2-6, H-8/H3-17, H2-11/H2-12, and H2-14/H2-15 (Fig. 2). The relative configurations at C-3, C-4, C-5, C-6, C-8, C-9, C-10, and C-13 were the same as those of macranthin A on the basis of NOESY data (Fig. 3). Thus, the structure of 3 was defined as 6β, 19-diacetoxy-9, 13-epoxy-3α, 15-dihydroxylabdan-7-one, and this compound was named 15-O-deacetylmacranthin A.
Compound 4 was isolated as a colorless gum, [α]D21 -22, and its 13C NMR and positive-ion HRESIMS data showing a protonated ion peak at m/z 455.2641 [M + H]+ established a molecular formula of C24H38O8, indicating six indices of hydrogen deficiency. The IR spectrum indicated the presence of hydroxy (3421 cm-1), ester carbonyl (1737 cm-1), and α, β-unsaturated carbonyl (1655 cm-1) functionalities. The 1H NMR data (Table 1) exhibited resonances for four tertiary methyl [δH 1.10, 1.25, 1.47, and 1.83 (s, each 3H)], two oxygenated methylene [δH 3.73 (m, 1H), δH 3.76 (m, 1H); δH 4.69 (d, 1H, J = 11.5 Hz), 4.82 (d, 1H, J = 11.5 Hz)], two oxygenated methine [δH 4.19 (d, 1H, J = 3.5 Hz); 5.08 (br s, 1H)], and two O-acetyl (δH 2.05 and 2.07, s, each 3H) groups. The 13C NMR spectrum showed 24 carbon resonances including six methyls, seven methylenes (two oxygenated), three methines (two oxygenated), and eight quaternary carbons (one ketocarbonyl, two ester carbonyl, two olefinic, and one oxygenated). The aforementioned data suggested that compound 4 is a highly oxygenated bicyclic labdane diterpenoid similar to macranthin F [2]. The 1H NMR and 13C NMR spectroscopic data (Table 1) were highly similar to those of macranthin F [2], with the exception of the deshielded H-3 resonance (δH 5.08; ΔδH +1.28) and the presence of an additional acetyl group (δH 2.05; δC 172.3, 20.7), indicating 4 to be the 3-O-acetyl derivative of macranthin F. This deduction was confirmed by the HMBC correlation from H-3 to the carbonyl carbon (δC172.3) of the acetyl group (Fig. 2). The 2D structure of 4 was further supported by 1H-1H COSY correlations of H2-1/H2-2, H2-2/H-3, H-5/H2-6, H-8/H3-17, H2-11/H2-12, and H2-14/H2-15 (Fig. 2). The relative configuration of 4 was established on the basis of the NOESY data (Fig. 3). The NOE correlations from H3-20 to H2-19 and from H-5 to H3-18 indicated that the A/B rings are trans-fused. NOE correlations of H-6/H-5, H-5/H-1α, and H-6/H3-18 indicated these protons are cofacial and α-oriented, while NOEs of H-3/H2-19, H2-19/H3-20 were consistent with these protons being β-cofacially oriented (Fig. 3). However, the configuration at C-13 could not be assigned. Accordingly, the structure of 4 was defined as 3α, 19-diacetoxy-6β, 13, 15-trihydroxylabd-8(9)-en-7-one, named 3-O-acetylmacranthin F.
Compounds 1-4 were evaluated for their inhibitory effects on the NO production in LPS-stimulated BV-2 microglial cells using Griess assay [2, 6, 7]. Compounds 1 and 4 showed weak inhibition of NO production with IC50 values of 35.8 ± 3.6 μmol/L and 48.6 ± 4.8 μmol/L, respectively. Indomethacin (IC50 = 30.6 ± 2.2 μmol/L) was used as a positive control. Compounds 2 and 3 were inactive (< 50% inhibition at 100 μmol/L, the highest concentration tested). In order to investigate whether the inhibitory activities of compounds 1 and 4 were due to cytotoxicity, their effects on cell proliferation/viability using the MTT method were measured. These two compounds (up to 100 μmol/L) did not show any cytotoxicity with LPS treatment for 24 h.
In summary, one cis clerodane-type (1) and three highly oxygenated labdane-type diterpenes (2-4) were isolated from the aerial parts of Leonurus macranthus. The cis-fusion of rings A/B is rarely encountered in clerodane-type diterpenoids. Compound 1 represents the first example of cis clerodane-type diterpene in the plants of Leonurus genus. Compounds 1 and 4 exhibited weak inhibition of NO in LPS-stimulated BV-2 microglial cells with IC50 values of 35.8 ± 3.6 μmol/L and 48.6 ± 4.8 μmol/L, respectively.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 81573572, 81530097) and New Century Excellent Talents in University (No. NCET-13-0693).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2017.11.033.
| [1] |
Z.Y. Yang, S.L. Pan, K.K. Huo, et al., Am. J. Chin. Med. 39 (2011) 411-422. DOI:10.1142/S0192415X11008919 |
| [2] |
Z. Huang, Z.X. Zhu, Y.T. Li, et al., J. Nat. Prod. 78 (2015) 2276-2285. DOI:10.1021/acs.jnatprod.5b00635 |
| [3] |
Z. Huang, H.X. Huo, Y. Ren, et al., Chin. Trad. Herbal Drugs 48 (2017) 1724-1792. |
| [4] |
J. Li, F.R. Fronczek, D. Ferreira, et al., J. Nat. Prod. 75 (2012) 728-734. DOI:10.1021/np3000156 |
| [5] |
H. Li, M.M. Li, X.Q. Su, et al., J. Nat. Prod. 77 (2014) 1047-1053. DOI:10.1021/np5001329 |
| [6] |
J. Li, K.W. Zeng, S.P. Shi, et al., Fitoterapia 83 (2012) 896-900. DOI:10.1016/j.fitote.2012.03.025 |
| [7] |
K.W. Zeng, J. Li, X. Dong, et al., Toxicol. Appl. Pharmacol. 273 (2013) 159-171. DOI:10.1016/j.taap.2013.08.028 |
| [8] |
F. Freiburghaus, A. Steck, H. Pfander, et al., J. Ethnopharmacol. 61 (1998) 179-183. DOI:10.1016/S0378-8741(98)00035-X |
| [9] |
H.D. Sun, Natural Product Chemistry-Diterpene Chemistry. Beijing: Chemical Industry Press, 2012.
|
| [10] |
Z.K. Liu, D.R. Wu, Y.M. Shi, et al., Chin. Chem. Lett. 25 (2014) 677-679. DOI:10.1016/j.cclet.2014.01.047 |
| [11] |
H. Wu, J. Li, F.R. Fronczek, et al., Phytochemistry 91 (2013) 229-235. DOI:10.1016/j.phytochem.2012.02.021 |
 2019, Vol. 30
2019, Vol. 30