b College of Chemistry and Chemical Engineering, Nantong University, Nantong 226019, China
Supramolecular chemistry [1], constructed by noncovalent interaction of monomer, is a chemical system with manifold merits such as high-dynamic, self-repairing, multiple stimulus response, and autonomic regulation. The development and research of supramolecular polymers has been the focus of research in supramolecular chemistry. Supramolecular polymers, which are driven by the coordination bonds, combination of π-π and hydrogen bond driving forces, and the non-covalent interactions, are a kind of polymer that is formed by linker [2]. Supramolecular polymers have three advantages: In the first place, the invertibility of the non-covalent interactions of the supramolecular polymer is beneficial to the modification and recovery of the polymer, and has a wide range of stimulation response capability. At the same time, supramolecular polymers have excellent environmental adaptability and resiliency compared to other types of polymers. And studies have found that supramolecular polymers can participate in covalent cross-linking to form shape memory materials. The study on the formation process of supramolecular polymers in water system shows that hydrophobic interactions, electrostatic interactions and ionic-dipole interactions are the key reasons for the formation of supramolecular polymers [3].
Macrocyclic molecules are an outstanding type of supramolecular hosts which can complex with miscellaneous guest molecules and construct multifunction assembly structure [4-6]. The weak noncovalent interaction is one of the kernel processes of existence and reproduction of beings. Therefore, bioscience investigators have been committed to studying and developing the supramolecular chemistry in aqueous solution [7].
When macrocycle-based water-soluble supramolecular polymers self-assemble in water, macrocyclic host molecules and guest molecules are normally self-assembled by hydrophobic effects and hydrogen bonds effect. The assembly planning of intermolecular hydrogen bond effect between water molecules are unique and powerful in aqueous. The proposed effect would certainly affect the hydrogen bond highly recognized between macrocyclic host molecules and guest molecules and then seep into the desirable self-assemble effect in aqueous. Nonetheless, macrocyclic host molecules can shield intermolecular hydrogen bond effect between water molecules preferable and then precede high-intensitive host-guest recognized effect due to the special structure of macrocyclic host molecules. Hydrophobic effect is special phenomenon in aqueous, and the important factors affecting the given hydrophobic effect are the proportion of water in the system, and the size, polarity, and shape of the solute molecule [8]. Hydrophobic effect can form subunits between hydrophobic groups of host-guest molecules which participated in host-guest recognize, and the formed subunits can further self-assemble to form supramolecular copolymer [9].
The water-soluble supramolecular polymer system exists extensively in nature and has special physiological functions and special microstructures [10]. What is more, water-soluble supramolecular polymers have both characteristics of a supramolecular polymer and a water-soluble polymer compound [11]. Water-soluble supramolecular polymers are stimuli-responsive and have good response to thermal, light, electrical, and chemical response. At the same time, water-soluble supramolecular polymers can also be used for the use of heterogeneous catalysts to reduce the problems of high selectivity to substrates, difficulty in recovery, high cost, and non-environmental protection in conventional heterogeneous catalysts [12-14]. Therefore, this review collates the macrocyclic water-soluble supermolecular polymer system.
2. Cyclodextrins based water-soluble supramolecular polymersCyclodextrin (CD) is a kind of cyclic oligomer which is constituted by D-(+)-pyrano-glucose units linked by α-1, 4-glycosidic bonds. Among them, CDs are including the most common α-, β-, and γ-CD, and the cavity diameters of them are 0.49, 0.62, and 0.80 nm, respectively. CDs are easy to functionalize and many reviews reported the functionalization of CD at present [15-18]. Natural and modified CDs are host macrocyclic molecules with inherent hydrophobic cavities and hydrophilic surface. They are host-guest effects locus which is certified [19-21]. Compared with other macrocycle molecules, CDs are easy to gain and innocuousness. CDs can form complex molecules and supramolecular structures in aqueous environment and solid state.
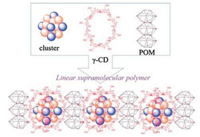
Nowadays, CD has been widely used in the field of materials and chemistry. For example, Moussawi [22] established a supramolecular hybridization system which is constituted by three components dawson-type polyoxometalate (POM) ([P2W18O62]6-), cationic electron-rich cluster ([Ta6Br12(H2O)6]2+), and γ-CD. They first explored the construction process of the supramolecular hybridization system. Research reveals that cationic electron-rich cluster closely embedded two γ-CD units to give the ditopic supramolecular cation. The ditopic supramolecular cation is acted as linker which extends the structure of polymers systems in next step. Soon afterwards, ditopic supramolecular cation and POM react together to produce three-component, well-ordered hybrid materials. The pre-binding form is verified. The formation of well-ordered hybrid materials dominated by supramolecular hydrogel or single crystal. Moussawi observed that the long-range order of the supramolecular polymers is ubiquity in the hydrogel hybrid material (Fig. 1).
|
Download:
|
| Fig. 1. Formation of supramolecular hybrid materials from polyoxometalate, cationic cluster, and γ-cyclodextrin. Reproduced according to Ref. [22]. | |
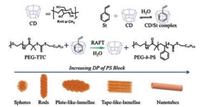
Besides that, a series of water-soluble supramolecular polymers contain traditional polymers were constructed. For example, Yuan et al. [23] found that CDs can be combined with styrene by polymerization-induced self-assembly (PISA) in the water system. The system can control the self-assembly in the polymer system by controlling the presence and proportion of components. When PISA occurred, the existence of CD is the crux to the formation of nanotubes. In reducing the CD/St ratios in the polymerization process, the resulting polymers are large-compound vesicles and large spheres rather than nanotubes. However, the addition of CD directly to self-assembly process of PEG-b-PS has no effect on the morphology of nanoparticles. Yuan et al. finally concluded that the CD only adjusts the shape of the PS chain during the polymerization period, and adjusts the mobility of the PS chain (Fig. 2).
|
Download:
|
| Fig. 2. Representation of RAFT dispersion polymerizationofthe cyclodextrin/styrene (CD/St) complex in water. PS = polystyrene, PEG = poly(ethylene glycol), DP = degree of polymerization. Reproduced according to Ref. [23]. | |
Jia et al. [24] designed and developed a kind of super-molecular pseudomonas polymer biomaterial with the advantages of water solubility, heat sensitivity, biocompatibility and degradation. First of all, the condensation of hydrophobic bile acid and hydrophilic polyethylene glycol produced polyurethanes with biodegradable ester bond. Then, β-CD will traverse through the polyurethanes and form polypseudorotaxanes in the water phase. The biomaterial can be used to identify cholic acid cells, and it can also change the phase inversion temperature by regulating the molar ratio of the β-CD and cholic acid units.
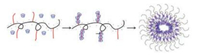
Meng [25] has synthesized a polymer hollow ball with good biodegradability, which can be used in drugs (especially sensitive and macromolecular enzymes or DNA) delivery systems and artificial cell domains. The preparation of the polymer hollow ball is based on the preparation strategy of the rod coil complex self-assembly, using alginate-graft-poly (ethylene glycol) as the object polymer and PEG as the grafting chain. The preparation strategy of the polymer hollow ball can effectively increase the space in the hollow core area of the polymer hollow sphere (Fig. 3).
|
Download:
|
| Fig. 3. Schematic illustration of the formation of the Alg-g-PEG/ a-CD hollow sphere. Reproduced according to Ref. [25]. | |
Potier [26] prepared hydroformylation catalysts with high reusability, hydrophobic Rh-catalyzed high olefin. Potier et al. combined the structural properties of thermal response poly(N-isopropylacrylamide) with the interface properties of randomly methylated β-cyclodextrin. It was found that there was no catalytic activity loss during the repeated use of the catalyst. Wang et al. [27] described a preparation strategy for a voltage responsive polymer nanoparticle. Among them, β-CD is used as crosslinking agent to connect poly(N-(2-hydroxyethyl)-acrylamide) (PHEAm) and 1, 8-diamino-3, 6-dioxaoctane bridged bis(ferrocene) (BisFc). When the nanoparticles are stimulated by external voltage, the structure of the polymer can be changed between the nanoparticles and the random coil.
Grana-Suarez [28] developed a multi-component super-molecular nanoparticle system developed based on the negatively charged polymer polymer poly(isobutyl-alt-maleic) acid. It is found that the size and stability of polymer particles in this system are controllable. The mechanism is based on the interaction between the host-guest effects and the repulsive electrostatic interaction. The host molecules in the systems are β-CD and the guest molecules are p-tert-butylphenyl group (Fig. 4).
|
Download:
|
| Fig. 4. Schematic representation of the assembly of the supramolecular nanoparticles from the host polymer PiBMA grafted withb-cyclodextrins (PiBMA-CD), the guest polymer PiBMA grafted with p-tert-butylphenyl groups (PiBMA-TBP), and AdPEG. Reproduced according to Ref. [28]. | |
3. Calixarene based water-soluble supramolecular polymers
Calixarene became the third generation supramolecular macrocyclic molecules due to its great potential of functionalization and high intensity of structure stability [29-31]. Compared with the first two generation supramolecular macrocyclic molecules, calixarene can adjust its cavity size as required [32]. Based on this property, the selectivity of supramolecular guest molecules, which can combine with calixarene host molecules, is greatly increasing. In addition, calixarene can form host-guest supramolecular system with ions and neutral molecules. Meanwhile, calixarene have good thermal stability, high melting point and good non-volatility. Therefore, calixarenes are widely used in various fields.
Supramolecular electrochemical response system is characteristic by polymerization of supramolecular polymers and no pollution of systems. Guo and others [33] were first designed and synthesized electrochemical response system which homoditopic calixarene (bis-SC4A) is host compounds and viologen (bis-MV4+) is guest compounds. In the system, host and guest molecule units can be gathered to become micron-scale linear polymers by electrochemical oxidationeffectively and the polymers can be decomposed to monomer by electrochemical reduction (Fig. 5).
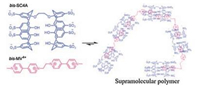
|
Download:
|
| Fig. 5. Schematic representation of self-assembling polymer formation by the iterative host-guest complexation between bis-SC4A and bis-MV4+. Reproduced according to Ref. [33]. | |
Orthogonal supramolecular polymer system is the research priority of supramolecular chemistry all the time. An orthogonal supramolecular polymer system which is stable and will not interfere with each other can enhance the responsiveness of supramolecular polymers system efficiently. On the other hand, orthogonal supramolecular polymer system can simulate the mechanism of production of biomaterial which is orthogonal self-assembly in nature. Zhao and others [34] developed a kind of linear supramolecular polymerization system based on aqueous environment. This kind of supramolecular polymer system possesses two kinds of supramolecular macrocyclic host molecules, cyclodextrinand calixarene and two kinds of supramolecular host-guest interactions. It is worth mentioning that the proposed supramolecular host-guest interactions are mutually orthogonal (Fig. 6).
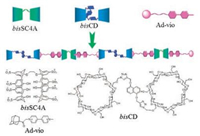
|
Download:
|
| Fig. 6. Structural illustration of bisSC4A, Ad-Vio, bisCD and supramolecular ternary polymer bisCD & Ad-Vio & bisSC4A. Reproduced according to Ref. [34]. | |
After research, appropriate selection of polymer receptors can enhance its function without affecting the function of the polymer. The conclusion was confirmed in the study of the supramolecular polymers system of calixarenes in Rambo group [35]. Rambo group prepared polymethyl methacrylate polymers (PMMA) which the neutral molecule receptor acts as side chain in the preliminary stage. They found that the PMMA polymers can extract some evaporates in aqueous effectivity. Nonetheless, the PMMA polymers cannot identify cesiumcations. Therefore, Rambo and others improved, designed and completed a kind of supramolecular polymer system which is established by methyl methacrylatepolymers (MMA) and benzocrown-6-calix[4]arene on the basis of PMMA polymers. The water-soluble supramolecular polymer system can recognize and extract the cesium cations effectively from aqueous medium. The extracted cesium cations were precipitated through combined with salt formation. Further research shows that the polymer system has higher selectivity to cesium cations compared with sodium cations and potassium cations.
Verdejo group [36] designed a calix[4]pyrrole receptor based supramolecular polymer system which can respond to two different stimuli. The proposed system can form gel adjusted by the concentration of host molecules and guest molecules and sodium cations and tetramethylammonium cations are requirements of gel forming. It was found that the proposed system can response favorable to sodium cation stimulates response and pH cation stimulates response (Fig. 7).
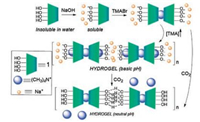
|
Download:
|
| Fig. 7. Schematic representation of the species and processes involved in the formation of hydrogels from aqueous solutions containing TMA, 1 and sodium hydroxide. Reproduced according to Ref. [36]. | |
4. Cucurbit[n]uril based water-soluble supramolecular polymers
Cucurbit[n]urils (CB[n]s), a family of macrocyclic compounds comprising n glycoluril units, have a hydrophobic cavity that is accessible through two identical carbonyl-fringed portals [37, 38]. The supramolecular chemistry of CB[n]s has captured wide attention in the scientific community. During the last two decades, a large variety of stimuli-responsive self-assemblies with interesting properties has been constructed based on the unique host-guest recognition properties of CB[n]s [39, 40]. However, the research on amphiphilic CB[n]s was relatively rare due to the difficulty in functionalization of CB[n]s.
Zhang [41] and others provided a new controllable supramolecular polymerization approach by host-guest interaction and photochemistry based on ABBA type monomer and cucurbit[8]uril monomer. The ABBA type monomer (1′, 1‴-(butane-1, 4-diyl)bis(1-(4-(phenyldiazenyl) benzyl)-[4, 4′-bipyridine]-1, 1′-diium)) is a guest with azobenzene-viologen-viologen-azobenzene structure array. A diav-4 monomer can be composed of a maximum of four CB[8] monomers to form a supramolecular polymer. Zhang and others can control the supermolecule polymerization by adjusting the molar ratio of supramolecular polymeric chain structure could of CB[8]/DIAV-4. In addition, azobenzene moiety can be photoisomerized under ultraviolet or visible light. The isomer ratio of azobenzene can also affect the degree of supramolecular polymerization, and the trans isomer of azobenzene is beneficial to the supermolecule polymerization. Therefore, by adjusting the molar ratio of the host and guest monomers or adjusting the isomer ratio of guest monomers after the competitive irradiation of lights can good control the molecular weight and polydispersity of supramolecular polymers. It provides a general method for controlling the structure of supermolecule polymerization and supermolecule polymer (Fig. 8).
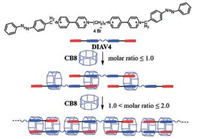
|
Download:
|
| Fig. 8. Schematic representation of controllable supramolecular polymerization by tuning the molar ratio of CB[8]/DIAV-4. Reproduced according to Ref. [41]. | |
Then they developed a general strategy for preparing polypseudorotaxanes with controllable antibacterial activity based on cationic polymers [42]. They construct an antibacterial regulation based on the host-guest interaction of CB[7] and the positive charge and hydrophobic component of ε-poly-L-lysine hydrochloride. The study shows that after adding CB[7], the number of aggregates in solution increased significantly, but the aggregate size did not increase. ε-poly-L-lysine hydrochloride is a typical small aggregates which contain many ε-poly-L-lysine hydrochloride, composed of polyelectrolytes. The addition of CB[7] in the system can effectively destroy the aggregates in order to release the ε-poly-L-lysine hydrochloride, thus forming the polypseudorotaxane into bulk. As a result, the mixture of CB[7] and cationic polymers is an antimicrobial polycyclic alkane. At the same time, adjusting the ratio of CB[7] and cationic polymer can effectively adjust the antibacterial efficiency of the system. This series of studies will enrich the application fields of polypseudorotaxanes and cationic polymers and the application has an important effect on the precise control of antibacterial activity.
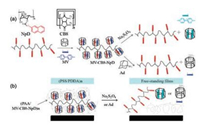
Ji et al. [43] used CB[8] base stimuli-responsive film as a sacrificial layer to prepare free-standing thin films. They first used an N, N'-dicyclohexylcarbodiimide-mediated coupling method to synthesize a dextran modified with a 2-naphthoxy group (NpD). And then, the doubly-charged binary complex of CB[8] and methyl viologen would proceed equimolar complexation in aqueous. Ji et al. transformed neutral NpD into a pseudo-polycation MV-CB[8]-NpD. Subsequently, the pseudo-polycation was assembly with poly(acrylic acid) (PAA) layer bylayer to stimulate the responsive membrane. The study shows that the Reversibility of the combination of the host-guest interaction of CB[8] provides the way to adjust the positive charge of MV-CB[8]-NpD and endow inherent stimuli responsiveness to the resulting multilayer films. This unique stimuli responsive multilayer film can trigger the effective triggered release of top thin films effectively under certain mild conditions. This study provides a simple and effective strategy for the realization of free-standing thin films, which can be used in the field of optics, electronics and biomaterials (Fig. 9).
|
Download:
|
| Fig. 9. Schematic of (a) the modified dextran with controlled charge properties through supramolecular complexation-decomplexation, and chemical structure of methyl viologen (MV) and its one-electron reduced form, 2-naphthoxy modified dextran (NpD), cucurbit[8]uril (CB[8]) and aminoadamantane (Ad); (b) the preparation of free-standing films. Reproduced according to Ref. [43]. | |
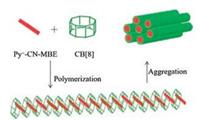
Park and others [44] designed a system based on a CB[8] host and a newly designed cyanostilbene guest. The cyanostilbene derivative does not emit fluorescence in aqueous solution, but it becomes high fluorescence, linear and rigid supramolecular polymer when after the host-guest interacting with CB[8]. The cyanostilbene derivative is a chromophoric monomer, which provides high binding affinity for CB[8] and water solubility, composed by a cyanostilbene backbone and 1-methylpyridinium moiety. The study showed that the formation of stilbene derivative of no cyano unit inhibited the supermolecule polymerization of CB[8], and partially quenched the fluorescence. Therefore, this supermolecular polymer with high purity fluorescence in pure water can be applied to biological fields with unique and amplification fluorescence modulation (Fig. 10).
|
Download:
|
| Fig. 10. Representation of Py+-CN-MBE@CB[8] polymer formation. Reproduced according to Ref. [44]. | |
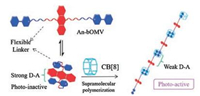
Chen and others successfully constructed the supermolecule photodegradationassemblies based on the host-guest interaction of CB[8] and photo-decomposable guest [45]. Among them, the pro-photoactive polymeric monomer is composed by 9, 10-dialkoxyanthracenes as electronrich cores and monocharged 4, 4-bipyridiniums (MV+) as electrondeficient terminals. Studies have shown that, under light conditions, the 9, 10-dialkoxyanthracenes section of An-bOMV geust can be slowly decomposed into anthraquinone and alkanol. After adding the CB[8], the photodecomposition effect of the system was significantly enhanced, accompanied by the degradation of the linear supramolecular polymer. This strategy provides a new way for the development of photosensitive polymer materials (Fig. 11).
|
Download:
|
| Fig. 11. Schematic illustrations of evolutions of D-A interactions and photoactivities induced by supramolecular polymerization. Reproduced according to Ref. [45]. | |
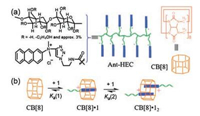
Abell [46] take advantage of a single emulsion droplet microfluidics technology, which by using the supramolecular host-guest assembly of the macrocyclic host CB[8] and the guest of anthracene-functionalized hydroxyethyl cellulose (Ant-HEC) polymers, for the preparation of supramolecular hydrogel microcapsules. Researches show that: in contrast to the process of building a microcapsule by droplet-in-droplet double emulsion method, the electrostatically-directed attraction of the supramolecular polymer in water and electrostatically-directed attraction can help to promote forming supramolecular hydrogel skins in a single emulsion. This interaction can be destroyed by introducing a competitive guest, thereby achieving the high molecular permeability triggered. The process of transforming the supramolecular hydrogel network from a non-covalent structure to covalent structure post-fabrication provides a mechanism for controlling the permeability of the microcapsule. This adds potential applications to self-assembled hydrogel microcapsules, such as those used in agricultural chemicals, nutrition or cosmetics (Fig. 12).
|
Download:
|
| Fig. 12. (a) Chemical structures of the components used in this study and their schematic representations: CB[8] and Ant-HEC. (b) Schematic illustration of the formation of homoternary complexes by accommodating two anthracene moieties in the cavity of CB[8]. Reproduced according to Ref. [46]. | |
5. Pillar[n]arene based water-soluble supramolecular polymers
Pillar[n]arenes are a new class of macrocyclic hosts containing electron-rich and hydrophobic cavities. Their repeating units are connected by methylene bridges at the para-positions, forming a unique rigid pillar architecture [47, 48]. They can be easily chemically modified, making them promising candidates for application in molecular recognition, supramolecular self-assembly, supramolecular polymers, etc. [49, 50]. However, the studies on pillararene-based macrocyclic amphiphilesare relatively rare, mainly due to the difficulties in the synthesis of nonsymmetrical pillararenes. To the best of our knowledge, to date pillararene based macrocyclic amphiphiles have been mostly constructed based on pillar[5]arenes because of the relatively easy availability of nonsymmetrical pillar[5]arenes.
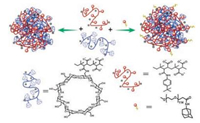
Our group prepared the first water soluble pillar[5]arene based supramolecular polymer by using the easily available water-soluble pillararene monomer (Fig. 13) [51]. Linear supramolecular polymers were efficiently constructed driven by host-guest interactions in water. By a combination of various techniques, including 1H NMR, 2D-NOESY, SEM and viscometry, it was demonstrated that the formation of supramolecular polymers was highly dependent on the monomer concentration. Moreover, a rod-like fiber was drawn from a high-concentration solution, providing direct evidence for the formation of a linear supramolecular polymer. Furthermore, supramolecular polymers showed reversible glue-sol phase transitions upon heating and cooling. Considering the easy availability and good water-soluble properties of the monomer, the present study provided a new and simple method to fabricate water-soluble supramolecular polymeric materials (Fig. 13).
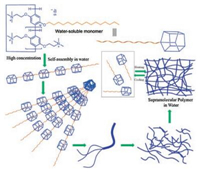
|
Download:
|
| Fig. 13. Cartoon representation of the self-assembly process of water soluble monomerpillar[5]arene in water. Reproduced according to Ref. [51]. | |
Then in 2017, our group [52] synthesized the first pillar[5]arene dimer and demonstrated the formation process of a fluorescent supramolecular polymer network is driven by hydrophobic and electrostatic interactions of carboxylate anion-functionalized tetraphenyl ethylene. Research shows that fluorescence intensity will increase rigidly with the participation of pillar[5]arene dimer and the poroper torsional conformation of carboxylate anion-functionalized tetraphenyl ethylene will be restrained by the host-guest effects. Then the carboxylate anion-functionalized tetraphenyl ethylene will be induced by proton to convert to acidity carboxylate anion-functionalized tetraphenyl ethylene, soon afterwards pillar[5]arene dimer will dissociation from the cavity. On account of the low solubleness of acidity carboxylate anion-functionalized tetraphenyl ethylene, fluorescence intensity of the network will more reinforce. Based on the pH-responsive ability of the host-guest effects of the pillar[5]arene, fluorescence intensity of the network can be adjusted by changing the pH of the solvents (Fig. 14).
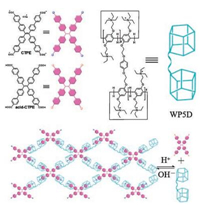
|
Download:
|
| Fig. 14. Chemical structure and schematic representation of CTPE, acid-CTPE, and WP5D and the reversible construction of the fluorescent supramolecular polymer network in water based on CTPE and WP5D. Reproduced according to Ref. [52]. | |
Furthermore, Huang and his team [53] have established a novel molecular recognition motif between a new heat-sensitive water-soluble pillar[7]arene (WP7) and an azobenzene derivative. The research shows that this new recognition motif has dual thermo-and photo-responsiveness in aqueous. Huang and his team constructed the first self-assembled vesicle pillararene-based supra-amphiphilic polypseudorotaxane by using this identification motif. Due to the hermoresponsiveness of WP7 and photoresponsiveness of the azobenzene unit, the reversible transformation between vesicles, which based on the self-assembled solid nanoparticles of a polymer skeleton and superhermaphroditic-poly pseudodeoxane self-assembly, is achieved by adjusting the temperature of the solution or UV-vis light. Therefore, vesicle calcein can be further used in water-soluble dye calcein molecules controlled release. This study provides a new method for the construction of functional supramolecular materials (Fig. 15).
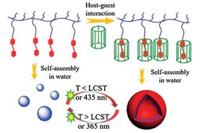
|
Download:
|
| Fig. 15. The illustration of the dual-responsive controlled assembly and disassembly of WP7/guest supramolecular vesicles. Reproduced according to Ref. [53]. | |
6. Conclusion
As described above, considerable progress has been achieved in the field of macrocycle-based water-soluble supramolecular polymers over the past decades. Supramolecular polymers can show traditional polymeric properties, although the molecular weights of them are usually smaller than the traditional ones and the noncovalent interactions to form supramolecular polymers are relatively weaker. The water soluble supramolecular polymers are formed in aqueous solution mainly via hydrophobic effect, ion-dipole interaction, electrostatic interaction and host-guest interactions. What is most interesting properties for supramolecular polymers are that the monomer of them can be diversified and involve various functional units with fluorescent emission, adjustable absorption, chirality, redox-feasibility, hydrophobicity, hydrophility, and so forth. And the supramolecular polymers are easy to be controllably responsive to external stimuli such as alternative light-irradiation, chemical or electrochemical redox, pH variance, heating and cooling, and so forth. Construction of hydrophilic supramolecular polymers in aqueous solution also aims to develop new polymeric materials besides constructing fascinating supramolecular structures. Cyclodextrin and cucurbituril based supramolecular polymerhydrogels, as new special soft materials, have been reported, some of which were provided with self-healing and shapememory properties. It should be noted that hydrophilic supramolecular polymers would be developed as promising biocompatible materials in the future, although their supramolecular structures still need be well designed and macroscopical properties such as machinability enhanced. Both novel concepts and effective methodology still need be proposed and developed to control the supramolecular structures of the polymers and to address their precise characterization. Moreover, the related dynamic study is still less during the process of supramolecular polymerization. Developing novel supramolecular polymers toward diverse applicable functionality, especially as self-healing soft materials, is a very interesting trend for the future.
AcknowledgmentsThis work was supported the National Natural Science Foundation of China (No. 21801139), Natural Science Foundation of Jiangsu Province (No. BK20180942), the Natural Science Foundation of Nantong University (No. 03083004), and the large instruments open foundation of Nantong university (No. KFJN1814).
| [1] |
B. Zheng, F. Wang, S. Dong, et al., Chem. Soc. Rev. 41 (2012) 1621-1636. DOI:10.1039/C1CS15220C |
| [2] |
J. Zhang, J. Zhu, C. Lu, et al., Polym. Chem. 7 (2016) 4317-4321. DOI:10.1039/C6PY00872K |
| [3] |
X. Yan, F. Wang, B. Zheng, et al., Chem. Soc. Rev. 41 (2012) 6042-6065. DOI:10.1039/c2cs35091b |
| [4] |
C. Xu, L. Xu, X. Ma, Chin. Chem. Lett. 29 (2018) 970-972. DOI:10.1016/j.cclet.2017.11.045 |
| [5] |
T. Xiao, W. Zhong, L. Zhou, et al., Chin. Chem. Lett. 30 (2019) 31-36. DOI:10.1016/j.cclet.2018.05.034 |
| [6] |
T. Xiao, L. Zhou, L. Xu, et al., Chin. Chem. Lett. (2018). DOI:10.1016/j.cclet.2018.05.039 |
| [7] |
X. Ma, T. He, Acc. Chem. Res. 47 (2014) 1971-1981. DOI:10.1021/ar500033n |
| [8] |
T. Aida, E.W. Meijer, S.I. Stupp, Science 335 (2012) 813-817. DOI:10.1126/science.1205962 |
| [9] |
D. Chandler, Nature 437 (2005) 640-647. DOI:10.1038/nature04162 |
| [10] |
D.S. Guo, T.X. Zhang, Y.X. Wang, et al., Chem. Commun. 49 (2013) 6779-6781. DOI:10.1039/c3cc41918e |
| [11] |
X. Ma, R. Sun, W. Li, et al., Polym. Chem. 2 (2011) 1068-1070. DOI:10.1039/c0py00419g |
| [12] |
X. Zheng, Q. Miao, W. Wang, et al., Chin. Chem. Lett. 24 (2013) 351-358. DOI:10.1016/j.cclet.2013.03.018 |
| [13] |
Q. Wang, M. Cheng, J.L. Jiang, et al., Chin. Chem. Lett. 28 (2017) 793-797. DOI:10.1016/j.cclet.2017.02.008 |
| [14] |
F. Sakai, Z.W. Ji, J.H. Liu, et al., Chin. Chem. Lett. 24 (2013) 568-572. DOI:10.1016/j.cclet.2013.04.027 |
| [15] |
S. Lay, X. Ni, H. Yu, et al., J. Sep. Sci. 39 (2016) 2321-2331. DOI:10.1002/jssc.v39.12 |
| [16] |
J. Guo, Y. Lin, Y. Xiao, et al., J. Pharm. Biomed. Anal. 130 (2016) 110-125. DOI:10.1016/j.jpba.2016.05.023 |
| [17] |
F.G. Adly, N.Y. Antwi, A. Ghanem, Chirality 28 (2016) 97-109. DOI:10.1002/chir.v28.2 |
| [18] |
M. Kryjewski, T. Goslinski, J. Mielcarek, Coord. Chem. Rev. 300 (2015) 101-120. DOI:10.1016/j.ccr.2015.04.009 |
| [19] |
G. Chen, M. Jiang, Chem. Soc. Rev. 40 (2011) 2254-2266. DOI:10.1039/c0cs00153h |
| [20] |
J. Szejtli, Chem. Rev. 98 (1998) 1743-1754. DOI:10.1021/cr970022c |
| [21] |
W. Saenger, J. Jacob, K. Gessler, et al., Chem. Rev. 98 (1998) 1787-1802. DOI:10.1021/cr9700181 |
| [22] |
M.A. Moussawi, N. Leclerc-Laronze, S. Floquet, et al., J. Am. Chem. Soc. 139 (2017) 12793-12803. DOI:10.1021/jacs.7b07317 |
| [23] |
X. Chen, L. Liu, M. Huo, et al., Angew. Chem. Int. Ed. 56 (2017) 16541-16545. DOI:10.1002/anie.201709129 |
| [24] |
Y.G. Jia, C. Malveau, M.A. Mezour, et al., Angew. Chem. Int. Ed. 55 (2016) 11979-11983. DOI:10.1002/anie.201605090 |
| [25] |
X.W. Meng, J. Qin, Y. Liu, et al., Chem. Commun. 46 (2010) 643-645. DOI:10.1039/B916224K |
| [26] |
J. Potier, S. Menuel, J. Lyskawa, et al., Chem. Commun. 51 (2015) 2328-2330. DOI:10.1039/C4CC09052G |
| [27] |
F. Wang, H. Pu, X. Che, Chem. Commun. 52 (2016) 3516-3519. DOI:10.1039/C5CC09984F |
| [28] |
L. Grana-Suarez, W. Verboom, J. Huskens, Chem. Commun. 50 (2014) 7280-7282. DOI:10.1039/C4CC03136A |
| [29] |
Y. Sun, Y. Yao, C.G. Yan, et al., ACS Nano 4 (2010) 2129-2141. DOI:10.1021/nn901412n |
| [30] |
F. Perret, A.W. Coleman, Chem. Commun. 47 (2011) 7303-7319. DOI:10.1039/c1cc11541c |
| [31] |
S. Sameni, C. Jeunesse, D. Matt, et al., Chem. Soc. Rev. 38 (2009) 2117-2146. DOI:10.1039/b900183b |
| [32] |
H. Iwamoto, S. Nishi, T. Haino, Chem. Commun. 47 (2011) 12670-12672. DOI:10.1039/c1cc14739k |
| [33] |
D.S. Guo, S. Chen, H. Qian, et al., Chem. Commun. 46 (2010) 2620-2622. DOI:10.1039/b925157j |
| [34] |
H.X. Zhao, D.S. Guo, L.H. Wang, et al., Chem. Commun. 48 (2012) 11319-11321. DOI:10.1039/c2cc34834a |
| [35] |
B.M. Rambo, S.K. Kim, J.S. Kim, et al., Chem. Sci. 1 (2010) 716-722. DOI:10.1039/c0sc00396d |
| [36] |
B. Verdejo, F. Rodriguez-Llansola, B. Escuder, et al., Chem. Commun. 47 (2011) 2017-2019. DOI:10.1039/c0cc04051g |
| [37] |
K. Kim, N. Selvapalam, Y.H. Ko, et al., Chem. Soc. Rev. 36 (2007) 267-269. DOI:10.1039/B603088M |
| [38] |
K.I. Assaf, W.M. Nau, Chem. Soc. Rev. 44 (2015) 394-418. DOI:10.1039/C4CS00273C |
| [39] |
A.I. Day, R.J. Blanch, A.P. Arnold, et al., Angew. Chem. Int. Ed. 41 (2002) 275-277. DOI:10.1002/1521-3773(20020118)41:2<275::AID-ANIE275>3.0.CO;2-M |
| [40] |
J. Zhang, R.J. Coulston, S.T. Jones, et al., Science 335 (2012) 690-694. DOI:10.1126/science.1215416 |
| [41] |
L. Yang, Y. Bai, X. Tan, et al., ACS Macro Lett. 4 (2015) 611-615. DOI:10.1021/acsmacrolett.5b00266 |
| [42] |
Z. Huang, H. Zhang, H. Bai, et al., ACS Macro Lett. 5 (2016) 1109-1113. DOI:10.1021/acsmacrolett.6b00568 |
| [43] |
D.D. Li, X.C. Chen, K.F. Ren, Chem. Commun. 51 (2015) 1576-1578. DOI:10.1039/C4CC07899C |
| [44] |
H.J. Kim, D.R. Whang, J. Gierschner, et al., Angew. Chem. Int. Ed. 55 (2016) 15915-15919. DOI:10.1002/anie.201609699 |
| [45] |
T. Qian, F. Chen, Y. Chen, et al., Chem. Commun. 53 (2017) 11822-11825. DOI:10.1039/C7CC07560J |
| [46] |
Z. Yu, J. Zhang, R.J. Coulston, et al., Chem. Sci. 6 (2015) 4929-4933. DOI:10.1039/C5SC01440A |
| [47] |
M. Xue, Y. Yang, X. Chi, et al., Acc. Chem. Res. 45 (2012) 1294-1308. DOI:10.1021/ar2003418 |
| [48] |
Y. Yao, X. Wei, Y. Cai, et al., J. Colloid Interface Sci. 525 (2018) 48-53. DOI:10.1016/j.jcis.2018.04.034 |
| [49] |
Y. Ma, X. Ji, F. Xiang, et al., Chem. Commun. 47 (2011) 12340-12342. DOI:10.1039/c1cc15660h |
| [50] |
Y. Yao, M. Xue, J. Chen, et al., J. Am. Chem. Soc. 134 (2012) 15712-15715. DOI:10.1021/ja3076617 |
| [51] |
B. Shi, D. Xia, Y. Yao, Chem. Commun. 50 (2014) 13932-13935. DOI:10.1039/C4CC06971D |
| [52] |
Y. Sun, J. Wang, Y. Yao, Chem. Commun. 53 (2016) 165-167. |
| [53] |
X. Chi, X. Ji, D. Xia, et al., J. Am. Chem. Soc. 137 (2015) 1440-1443. DOI:10.1021/ja512978n |
 2019, Vol. 30
2019, Vol. 30 

