b Department of Chemistry, Maynooth University, National University of Ireland, Maynooth, Ireland;
c Key Laboratory of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China
Currently, the energy crisis is a worldwide problem and searching for clean and economical energy sources is a global challenge. Inspired by nature, many scientists are attempting to solve this problem by taking inspiration from photosynthesis, a natural process that generally takes place in green plants to produce clean energy [1, 2]. As we know, photosynthesis plays an important role in our lives [3, 4], where this efficient process results in photon-harvesting through a large number of closely packed chlorophyll molecules inside pigment-protein complexes to produce energy and oxygen. The profound efficiency of this process helps organisms to survive even under low-light conditions. As we can see, the photosynthesis process can capture, transfer, and store solar energy effectively. Thus, scientists are committed to fabricating artificial light harvesting systems (LHSs) to mimic this natural process. Unfortunately, conventional chromophoreshave a high propensity towards aggregation at high concentration, which is detrimental for photoluminescence [5]. Consequently, exploiting approaches to maximize the density of chromophores while minimizing self-quenching are still a big challenge in the construction of LHSs. With the rapid development of supramolecular chemistry, more and more effort has focused on developing artificial LHSs by self-assembly.
Macrocycle-based host-guest interactions have played an important role in supramolecular chemistry due to their reversible properties [6-10]. Introduction of reversible host-guest interactions can endow materials with fascinating properties, such as stimuli responsiveness, self-healing and adaptability. Macrocylic hosts are important building blocks in supramolecular chemistry and usually refer to crown ethers, calixarenes, cyclodextrins (CD), cucurbiturils (CB), and pillar[n]arenes. Employing such hosts has turned out to be a useful approach to maximise fluorescence intensity whereby encapsulation of a chromophoric guests prevents the chromophores from stacking [5]. In the development of the optimal LHS, three critical factors need to be considered: (a) strong and broad absorption ability across the full spectrum of sunlight, (b) efficient and fast energy transfer from antenna compounds to a reaction center, and (c) a high degree of photostability. In this mini-review, we will introduce recent works on LHSs constructed by macrocycle-based host-guest interactions.
2. Light-harvesting systems based on host-guest interactions 2.1. LHSs constructed by pillar[n]arenesDue to the efficiency of the solar energy storage mechanism in photosynthesis, constructing artificial LHSs is an emerging scientific research area which has attracted much attention. However, the construction of highly efficient LHSs in aqueous solution is still a major challenge. As a new generation of macrocylichost, pillar[n]arenes exhibit excellent host-guest recognition interactions [11-16]. Pillar[n]arenes have numerous phenolic units on both sides, leading them to be analogous to CDs and also soluble in H2O [17]. It should be noted that the symmetrical pillar structure and easy modification of pillar[n]arenes endows them with the ability to bind a variety of guests, such as neutral guests, cationic guests, anionic guests, rigid guests, flexible chain guests, hydrophobic guests, and hydrophilic guests [18, 19]. In this part of the review, we will discuss recent reports on efficient LHSs constructed from water-soluble pillar[n]arene.
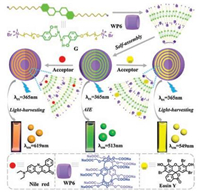
In 2018, Wang et al. reported new LHSs in aqueous solution (Fig. 1) [20]. In recent years, they have been committed to exploiting functional materials by supramolecular host-guest interactions, such as supramolecular polymers [9, 21-29], drug delivery systems [30-33], and functional organogels and hydrogels [34-36]. In this work, this system was based on the supramolecular self-assembly of a water-soluble pillar[6]arene (WP6), a salicylaldehyde azine derivative (G), and two types of fluorescence dye, nile red (NiR) or eosin Y (ESY). Salicylaldehyde azine derivative G is emissive when aggregated through the combined mechanism of aggregation induced emission (AIE) and excited-state intramolecular proton transfer (ESIPT). In this work, WP6 first formed a stable host-guest complex with G before spherical nanoparticles could be formed from WP6-G via hydrophobic interactions. It should be noted that WP6 can not only remarkably lower the critical aggregation concentration (CAC) of G but can also significantly improve the AIE of G. According to our experiments, in the presence of WP6, the fluorescence intensity of G increased up to a maximum of 30 times and the CAC value of G decreased at least 28-fold. The morphology and size of the nanoparticles were studied by dynamic light scattering (DLS), transmission electron microscopy (TEM), and scanning electronic microscopy (SEM). The DLS showed the well-defined aggregates formed with a narrow size distribution and had an average diameter of 109 nm. The TEM and SEM photos displayed spherical morphology with diameters about 100 nm, which was consistent with the DLS results.
|
Download:
|
| Fig. 1. Illustration of the self-assembly of pillar[6]arene-based aqueous light-harvesting systems. Copied with permission [20]. Copyright 2018, Wiley Publishers. | |
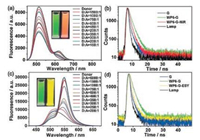
The spherical nanoparticles play the role of a donor for the artificial LHSs, and the hydrophobic fluorescent dye ESY or NiR, which are loaded within the hydrophobic interior of the nanoparticles, act as acceptors (Fig. 1). The selection of NiR and ESY as fluorescent acceptors allows both of their absorption bands to largely overlap with the fluorescence band of the WP6-G. Fluorescence spectraand fluorescence decay profiles show that energy transfer takes place from the WP6-G to both of the encapsulated NiR and ESY fluorophores (Fig. 2). As shown in Fig. 2a, with the gradual addition of NiR to WP6-G, the fluorescence intensity of the WP6-G decreased, while the fluorescence emission of NiR (acceptor) increased when excited at 365 nm. By contrast, the emission of free NiR was negligible upon excitation at 365 nm or even at 580 nm. Furthermore, fluorescence decay experiments showed the decay curve of G (Fig. 2b, blue line) was fitted as a double exponential decay with fluorescence lifetimes of τ1 = 0.32 ns and τ2 = 1.62 ns, both of which were related to the stacked G. For the WP6-G (Fig. 2b, green line), the fluorescence lifetimes increased to τ1 = 0.70 ns and τ2 = 1.95 ns due to WP6-induced aggregation of G. For the WP6-G-NiR assembly (Fig. 2b, red line), the fluorescence lifetimes decreased to τ1 = 0.61 ns and τ2 = 1.69 ns, confirming the energy transfer occurs from WP6-G donor to NiR acceptor. These results indicate that energy transfer takes place from the WP6-G assembly to the encapsulated NiR. Further studies showed that ESY can also serve as an excellent acceptor (Figs. 2c and d). It should be noted that the antenna effect at the best mixing ratio was calculated to be 25.4 for NiR and 28.0 for ESY, indicating that the nanoparticles act as an excellent light harvesting antenna in an aqueous environment. These examples of aqueous artificial LHSs provide a versatile platform for mimicking photosynthesis.
|
Download:
|
| Fig. 2. (a) Fluorescence spectra of WP6-G ([WP6] = 5 × 10-6 mol/L, [G] = 2 × 10-5 mol/L) in water with different concentrations of NiR. Inset: photographs of WP6-G and WP6-G-NiR([WP6] = 5 × 10-6 mol/L, [G] = 2 × 10-5 mol/L, [NiR] = 1.3 × 10-7 mol/L). (b) Fluorescence decay profiles of G (blue line), WP6-G (green line), and WP6-G-NiR (red line) ([WP6] = 5 × 10-6 mol/L, [G] = 2 × 10-5 mol/L, [NiR] = 1.3 × 10-7 mol/L). (c) Fluorescence spectra of WP6-G ([WP6] = 5 × 10-6 mol/L, [G] = 2 × 10-5 mol/L) in water with different concentrations of ESY. Inset: photographs of WP6-G and WP6-G-ESY ([WP6] = 5 × 10-6mol/L, [G] = 2 × 10-5 mol/L, [ESY] = 1 × 10-7 mol/L). (d) Fluorescence decay profiles of G(blue line), WP6-G (green line), and WP6-G-ESY (yellow line) ([WP6] = 5 × 10-6 mol/L, [G] = 2 × 10-5 mol/L, [ESY] = 1 × 10-7 mol/L). Copied with permission [20]. Copyright 2018, Wiley Publishers. | |
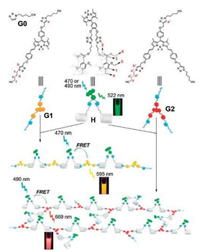
Prior to the above work, Wang et al. first disclosed an artificial LHS constructed by pillar[n]arenes in 2015 [37]. In this work, AA/BB-type and A2/B3-type FRET-capable supramolecular polymers based on a borondipyrromethene (BODIPY) bridged pillar[5]arene dimer and two BODIPY derivative guests were initially prepared (Fig. 3). The application of these supramolecular polymers in mimicking the LHS of natural photosynthesis was studied. In this work, the obtained supramolecular polymers displayed very strong absorption across a broad spectroscopic range from 300 to 700 nm and showed slightly different FRET effects in organic solution. A series of techniques were employed to characterize the supramolecular polymers, such as 1H NMR, DOSY, SEM, and UV-visabsorption. Furthermore, the energy transfer was confirmed by fluorescence titration experiments. The FRET efficiencies were calculated to be 51% for G1-H and 63% for G2-H due to the high complexation stability of the host-guest interaction. This case developed a novel supramolecular model for mimicking the LHS and exhibited potential applications in functional optoelectronic materials.
|
Download:
|
| Fig. 3. Chemical structures of H, G1, G2 and G0 and the cartoon representation of the construction of two kinds of FRET-capable functional supramolecular polymers. Copied with permission [37]. Copyright 2015, the Royal Society of Chemistry. | |
In 2016, Diao et al. reported stimulus-responsive light-harvesting complexesbased on the pillar[5]arene-induced co-assembly of β-carotene (β-CAR) and chlorophyll [38]. In this work, they first studied the host-guest interaction between water-soluble carboxyl-modified pillar[5]arene (WP5) and β-CAR. β-CAR could bind WP5 in water to achieve WP5⊃β-CAR complexation (WCC) via the hydrophobic effect, which was supported by NMR, Raman spectroscopy, fluorescence spectra and FT-IR spectroscopy (Fig. 4). WCCs were then employed as building blocks in the construction of light-harvesting antenna complexes (LHCs). After the as-prepared solutions were aged for 7 days, orange aggregates appeared in the solutions, which were determined to be hollow microspheres (HMSs). Interestingly, HMSs can provide a better chemical system in terms of stability without the loss of bioavailability. Moreover, they also prepared similar 'tadpole-like' host-guest complexes by using chlorophyll-b (Chl-b) instead of β-CAR. Based on the interesting properties showed by natural chlorophyll/carotenoid complexes in photosynthesis, Chl-b was selected as the co-assembly factor participating in the preparation process with WCC, and a similar suprastructure of Chl-b-containing LHC was built. These hydrophilic complexes displayed a series of unusual properties, including spontaneous growth, fusion, pH responsiveness and even some photocatalytic activity. The reported strategy paves a new avenue for the study of the origins of the bioenergysystem in living lives.

|
Download:
|
| Fig. 4. Structural model of building blocks. (a) LHC containing Chl-b formed by (b) WP5, (c) β-CAR and (d) Chl-b. For clarity, the β-CAR-based hydrophobic interior layer is orange, and the WP5-based hydrophilic exterior layer is green. Copied with permission [38]. Copyright 2016, Nature Publishing Group. | |
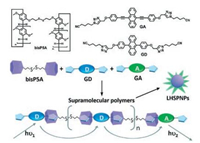
In 2018, Yang et al. reported artificial light-harvesting nanoparticles constructed by pillar[5]arene and anthracene modified donors and acceptors through supramolecular host-guest interactions [39]. Previously, some excellent LHSs based on other principles were also reported [40, 41]. In this work, they first prepared ternary supramolecular polymers (GD + GA + bisP5A) formed by host-guest interaction, which could be used to prepare water-dispersible nanoparticles (LHSPNPs) by using the microemulsion method (Fig. 5). LHSPNPs showed efficient energy transfer and high light harvesting ability because of the steric bulk of pillar[5]arene suppressing the self-quenching of the chromophores. Concomitantly, the energy transfer from donor GD to acceptor GA was maximized, affording highly emissive materials. GA is a green emissive diphenylethynylanthracene derivative while GD is a blue emissive diphenylanthracene derivative. The cyanoalkyltriazole substituent has strong binding affinity for pillar[5]arene with a binding constant of 1.2 × 104 L/mol in CHCl3, promoting the formation of the supramolecular polymers by host-guest interactions. Intriguingly, in contrast to nanoparticles formed from the ternary supramolecular polymers, under identical experimental conditions, GD alone formed nanosheets. The authors suspected this might be due to the π-π stacking of diphenylanthracene aromatic rings and hydrophobic interactions. However, in SPNPs, bulkiness provided by the pillar[5]arenes disrupted π-π stacking interactions, suppressing self-quenching and enhancing emission. LHSPNPs which were irradiated at 378 nm emitted at 494 nm. Time-resolved fluorescence measurements were employed to prove the energy transfer from GD to GA. As a result, the antenna effect reached a factor of 22 for the LHSPNPs containing a 0.5:99.5 M ratio of GA to GD. The approach described in this work may inspire the development of new luminescent materials.
|
Download:
|
| Fig. 5. Chemical structures of disulfide-bridged bispillar[5]arene (bisP5A) and the guest molecules (energy donor: GD and energy acceptor: GA); cartoon representations of bisP5A, GD, GA, their supramolecular polymers and the light-harvesting supramolecular polymeric nanoparticles(LHSPNPs); and the schematic light-harvesting paths in the LHSPNPs. Copied with permission [39]. Copyright 2018, the Royal Society of Chemistry. | |
2.2. LHSs constructed by calix[n]arenes
Development of broad-spectrum tunable photoluminescent nanomaterials is receiving much research interest due to their wide applications. To this end, LHSs involving energy migration can be exploited for the generation of multiple fluorescent emissions by tuning the energy-transfer efficiency between donor-acceptor components. In 2016, Guo et al. reported the design of a modular light-harvesting platform (Fig. 6) from calixarene amphiphiles, a typical class of macrocyclic amphiphiles, where the noncovalent positioning of donors and acceptors can be discretely addressed, in this example, reliant on self-sorting encapsulation and entrapment, respectively [42]. They first prepared amphiphilic calix[n]arenes (AmCnAs, n = 4 and 5) by decorating hydrophilic choline groups at the upper rim and hydrophobic alkyl chains at the lower rim, exhibiting unique superiority in self-assembly and host-guest recognition. DLS measurements reveal that both AmC4A and AmC5A form large-sized aggregates with averaged diameters of 149 nm and 79 nm, respectively. TEM and SEM photos of AmC5A display the spherical-like morphology with similar size to DLS results. It should be noted that the size of the amphiphilic assembly of AmC4A increased to 602 nm upon addition of 1-anilino-8-naphthalenesulfonate (1, 8-ANS), while that of AmC5A remains constant upon encapsulation of 1, 8-ANS. Thus they chose AmC5A to construct the light-harvesting platform. Then 1, 8-ANS and 4, 7-bis(thien-2-yl)-2, 1, 3-benzothiadiazole (DBT) were used as the donor/acceptor pair on account of the discrete addressability, spectrum overlap, and broad-spectrum tenability. Varying the molar ratio between donor and acceptor allows for the fine-tuning of the energy transfer process and generates broad-spectrum outputs, suggesting useful application as fluorescent inks with capability of encryption coding. Importantly, this case provides a model for light-harvesting nanomaterials from macrocyclic amphiphiles featuring full modularity and facile integration.

|
Download:
|
| Fig. 6. Schematic illustration of operating principle of the light-harvesting platform based on AmCnAs. Copied with permission [42]. Copyright 2016, Wiley Publishers. | |
2.3. LHSs constructed by cyclodextrins
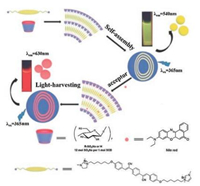
As is clear from the above examples, two necessary factors should be taken into consideration when constructing artificial LHSs: (a) the donor need to be densely packed without an obvious self-quenching effect, and (b) the ratio of donor to acceptor need to be high. Cyclodextrins are usually employed to construct light emission materials [43]. In 2017, Liu et al. reported a highly efficient aqueous LHS fabricated from the supramolecular self-assembly of an oligo(phenylenevinylene) derivative (OPV-I), sulfato-β-cyclodextrin (SCD), and nile red(NiR) (Fig. 7) [44]. The authors listed three advantages for this system: (a) at high concentration, OPV-I shows good AIE properties instead of the aggregation-caused quenching (ACQ) effect, which enables OPV-I to serve as a good donor; (b) the SCD macrocycle greatly lowers the CAC of OPV-I via host-guest interactions, improving the AIE properties of OPV-I and enabling good water solubility of the obtained LHS; and (c) NiR is loaded in the hydrophobic layer of the OPV-I/SCD nanoparticles and serves as a good acceptor. Consequently, the obtained OPV-I/SCD/NiR system exhibits a very high antenna effect, energy-transfer efficiency, and donor/acceptor ratio. Moreover, the antenna effect can take place in the presence of a trace amount of acceptor (donor/acceptor = 1500:1). In this case, the macrocylic host SCD also played an important role. With the addition of SCD, the fluorescence intensity of OPV-I increased 3.7 times. According to fluorescence studies, the antenna effect was determined to be 32.5 at a donor/acceptor ratio of 125:1, indicating that it was similar to a natural light-harvesting system.
|
Download:
|
| Fig. 7. Illustration of the construction of the light-harvesting system by Liu and co-workers. Reproduced with permission [44]. Copyright 2017, Wiley Publishers. | |
In 2016, Zhou et al. reported the hierarchical self-assembly of a dandelion-like supramolecular polymer (DSP) into nanotubes for application in LHSs (Fig. 8) [45]. The DSPs possess a "sphere-star-parachute" topological structure consisting of a spherical hyperbranched core and many parachute-like arms. This architecture was constructed by host-guest complexation between a β-cyclodextrin-endcapped hyperbranched multi-arm copolymer (host) and functionalized adamantanes with each having three alkyl chains (guests). The obtained DSPs can further self-assemble into nanotubes in aqueous solution in a hierarchical manner from vesicles to nanotubes. Subsequently, they use the bilayer nanotubes to construct LHSs in water. The LHS was fabricated by the incorporation of hydrophobic 4-(2-hydroxyethylamino)-7-nitro-2, 1, 3-benzoxa-diazole (NBD) as a donor inside the hyperbranched core of the nanotube and the hydrophilic rhodamine B (RB) as the acceptor immobilized on the nanotube surface. The resulting nanotube LHS displays surprisingly high energy transfer efficiency (above 90%) in water.

|
Download:
|
| Fig. 8. Preparation and self-assembly processes of DSPs. Copied with permission [45]. Copyright 2016, Wiley Publishers. | |
2.4. LHSs constructed by crown ethers
In 2017, Ye et al. reported a highly efficient FRET process from aggregation-induced emission to BODIPY emission based on host-guest interaction for mimicking the LHS [46]. In this work, AIE luminogen tetraphenylethene (TPE) was chosen to combine with crown ether moieties as a host (M1) and energy donor in FRET system (Scheme 1). Meanwhile, a BODIPY derivative containing benzylamino group as the guest (M2) and energy acceptor (Scheme 1). M1 becomes highly emissive in the aggregated state with a gradual increase in the fraction of a poor solvent. By contrast, M2 showed no remarkable emission intensity enhancement upon the addition of a poor solvent. Spectroscopic studies showed that the emission range of donor TPE (400-570 nm) almost coincided with the absorption range of acceptor BODIPY (430-530 nm). With gradual addition of M2, the AIE emission peak of M1 at 465 nm decreased gradually, while the emission peak of M2 at 510 nm appeared and then increased markedly, signifying the formation of a new complex between M1 and M2. From fluorescence titration experiments, the FRET efficiency was calculated to be 93% for this host-guest system.

|
Download:
|
| Scheme 1. FRET donor (host) M1 and acceptor (guest) M2. | |
2.5. LHSs constructed by cucurbiturils
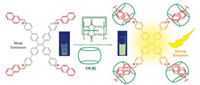
Cucurbit[8]uril (CB[8]) have attracted much research attention due to their ability to encapsulate two naphthalene moieties inside their internal cavity to form a ternary complex with high association constants [47-51]. In 2016, Ni et al. reported the fabrication of tunable luminescent materials in aqueous solution by employing a facile cucurbit[8]uril-based supramolecular approach [52, 53]. This strategy provided a toolbox for producing cyan, yellow, green, and white fluorescent emissions. In the same year, Xing et al. reported a simple strategy by mixing a naphthyl-substituted TPE derivative and CB[8] in aqueous solution to construct supramolecular hyperbranched polymers (Fig. 9) [54]. The host-guest binding between CB[8] and TPE restricted intramolecular rotation and non-radiative relaxation processes, resulting in strong emission from TPE in dilute solution. By employing the spherical aggregates made from the supramolecular polymers as energy donors and eosin Y disodium salt (EY) as an energy acceptor, they have constructed highly efficient LHSs in aqueous solution. Notably, significant fluorescence of the acceptor was observed even at extremely high donor/acceptor ratios (up to 200 000:1), indicating the highly efficient energy transfer between TPE-CB[8] and EY.
|
Download:
|
| Fig. 9. Illustration of the formation of supramolecular hyperbranched polymers through self-assembly of TPE and CB[8]. Reprinted with permission [54]. Copyright 2017, Elsevier Publishers. | |
Recently, Park et al. demonstrated a new system of light-harvesting supramolecular block copolymers in water by using CB[8] [55]. They prepared finely color-tuned supramolecular homopolymers comprising a CB[8] host and different cyanostilbene guests emitting blue, green, yellow, and red fluorescence, respectively, to realize CB[8]-based supramolecular block copolymers generating an artificial light-harvesting system in water. In this system, the light-harvesting supramolecular block copolymers show three advantages: (a) broad absorption, (b) enhanced exciton mobility through the polymerized cyanostilbene materials with high fluorescence quantum yields, and (c) stable supramolecular nanobundle formation in water.
3. Conclusions and outlookIn summary, developments in the construction of artificial light-harvesting systems (LHSs) based on macrocycle-based host-guest interactions are highlighted in this mini-review. The macrocycles used in constructing LHSs cover a diverse range of molecular scaffolds, such as calix[n]arenes, cyclodextrins, crown ethers, cucurbiturils, and especially the pillar[n]arenes. Moreover, the type of materials employed to fabricate LHSs is also diverse, ranging from linear and hyperbranched supramolecular polymers to nanoparticles. These varied examples have greatly enriched the field of artificial LHSs, however, it is worth noting that the described examples herein is not an exhaustive list but provides some inspiration for the future study of new LHSs. In the future, we anticipate that more and more highly efficient artificial LHSs driven by host-guest interactions will be reported and the field as a whole has a 'bright' future.
AcknowledgmentsWe gratefully thank the financial support of the National Natural Science Foundation of China (No. 21702020) and Maynooth University.
| [1] |
S. Kundu, A. Patra, Chem. Rev. 117 (2017) 712-757. DOI:10.1021/acs.chemrev.6b00036 |
| [2] |
G.D. Scholes, G.R. Fleming, A. Olaya-Castro, et al., Nat. Chem. 3 (2011) 763-774. DOI:10.1038/nchem.1145 |
| [3] |
T. Kondo, A. Pinnola, W.J. Chen, et al., Nat. Chem. 9 (2017) 772-778. DOI:10.1038/nchem.2818 |
| [4] |
N.E. Holt, D. Zigmantas, L. Valkunas, et al., Science 307 (2005) 433-436. DOI:10.1126/science.1105833 |
| [5] |
F. Biedermann, E. Elmalem, I. Ghosh, et al., Angew. Chem. Int. Ed. 51 (2012) 7739-7743. DOI:10.1002/anie.201202385 |
| [6] |
M. Zhang, X. Yan, F. Huang, et al., Acc. Chem. Res. 47 (2014) 1995-2005. DOI:10.1021/ar500046r |
| [7] |
S. Dong, B. Zheng, F. Wang, et al., Acc. Chem. Res. 47 (2014) 1982-1994. DOI:10.1021/ar5000456 |
| [8] |
T. Xiao, S.-L. Li, Y. Zhang, et al., Chem. Sci. 3 (2012) 1417-1421. DOI:10.1039/c2sc01004f |
| [9] |
S.L. Li, T. Xiao, C. Lin, et al., Chem. Soc. Rev. 41 (2012) 5950-5968. DOI:10.1039/c2cs35099h |
| [10] |
L. Qin, P.F. Duan, M.H. Liu, Chin. Chem. Lett. 25 (2014) 487-490. DOI:10.1016/j.cclet.2013.12.019 |
| [11] |
Q. Wang, M. Cheng, L. Tian, et al., Polym. Chem. 8 (2017) 6058-6063. DOI:10.1039/C7PY01096F |
| [12] |
T. Ogoshi, T.A. Yamagishi, Y. Nakamoto, Chem. Rev. 116 (2016) 7937-8002. DOI:10.1021/acs.chemrev.5b00765 |
| [13] |
N.L. Strutt, H. Zhang, S.T. Schneebeli, et al., Acc. Chem. Res. 47 (2014) 2631-2642. DOI:10.1021/ar500177d |
| [14] |
M. Xue, Y. Yang, X. Chi, et al., Acc. Chem. Res. 45 (2012) 1294-1308. DOI:10.1021/ar2003418 |
| [15] |
D. Cao, Y. Kou, J. Liang, et al., Angew. Chem. Int. Ed. 48 (2009) 9721-9723. DOI:10.1002/anie.200904765 |
| [16] |
X.S. Hu, H.M. Deng, J. Li, et al., Chin. Chem. Lett. 24 (2013) 707-709. DOI:10.1016/j.cclet.2013.05.008 |
| [17] |
Y. Ma, X. Ji, F. Xiang, et al., Chem. Commun. 47 (2011) 12340-12342. DOI:10.1039/c1cc15660h |
| [18] |
T. Ogoshi, T.A. Yamagishi, Y. Nakamoto, Chem. Rev. 116 (2016) 7937-8002. DOI:10.1021/acs.chemrev.5b00765 |
| [19] |
Y. Wang, G. Ping, C. Li, Chem. Commun. 52 (2016) 9858-9872. DOI:10.1039/C6CC03999E |
| [20] |
S. Guo, Y. Song, Y. He, et al., Angew. Chem. Int. Ed. 57 (2018) 3163-3167. DOI:10.1002/anie.201800175 |
| [21] |
Q. Wang, M. Cheng, L. Tian, et al., Polym. Chem. 8 (2017) 6058-6063. DOI:10.1039/C7PY01096F |
| [22] |
Q. Wang, M. Cheng, J.L. Jiang, et al., Chin. Chem. Lett. 28 (2017) 793-797. DOI:10.1016/j.cclet.2017.02.008 |
| [23] |
X.Y. Hu, T. Xiao, C. Lin, et al., Acc. Chem. Res. 47 (2014) 2041-2051. DOI:10.1021/ar5000709 |
| [24] |
T. Xiao, X. Feng, Q. Wang, et al., Chem. Commun. 49 (2013) 8329-8331. DOI:10.1039/c3cc44525a |
| [25] |
T. Xiao, X. Feng, S. Ye, et al., Macromolecules 45 (2012) 9585-9594. DOI:10.1021/ma302459n |
| [26] |
Y. Guan, M. Ni, X. Hu, et al., Chem. Commun. 48 (2012) 8529-8531. DOI:10.1039/c2cc33943a |
| [27] |
S.L. Li, T. Xiao, W. Xia, et al., Chem.-Eur. J. 17 (2011) 10716-10723. DOI:10.1002/chem.201100691 |
| [28] |
S.L. Li, T. Xiao, Y. Wu, et al., Chem. Commun. 47 (2011) 6903-6905. DOI:10.1039/c1cc12003d |
| [29] |
S.L. Li, T. Xiao, B. Hu, et al., Chem. Commun. 47 (2011) 10755-10757. DOI:10.1039/c1cc14559b |
| [30] |
X.Y. Hu, L. Wang, Supramol. Chem. (2017) 1-3. |
| [31] |
Y. Cao, Y. Li, X.Y. Hu, et al., Chem. Mater. 27 (2015) 1110-1119. DOI:10.1021/cm504445r |
| [32] |
Y. Cao, X.Y. Hu, Y. Li, et al., J. Am. Chem. Soc. 136 (2014) 10762-10769. DOI:10.1021/ja505344t |
| [33] |
Q. Duan, Y. Cao, Y. Li, et al., J. Am. Chem. Soc. 135 (2013) 10542-10549. DOI:10.1021/ja405014r |
| [34] |
X. Wu, Y. Yu, L. Gao, et al., Org. Chem. Front. 3 (2016) 966-970. DOI:10.1039/C6QO00197A |
| [35] |
M. Ni, N. Zhang, W. Xia, et al., J. Am. Chem. Soc. 138 (2016) 6643-6649. DOI:10.1021/jacs.6b03296 |
| [36] |
C.H. Yao, Q. Sun, W. Xia, et al., J. Organomet. Chem. 847 (2017) 68-73. DOI:10.1016/j.jorganchem.2017.03.042 |
| [37] |
L.B. Meng, D. Li, S. Xiong, et al., Chem. Commun. 51 (2015) 4643-4646. DOI:10.1039/C5CC00398A |
| [38] |
Y. Sun, F. Guo, T. Zuo, et al., Nat. Commun. 7 (2016) 12042-12054. DOI:10.1038/ncomms12042 |
| [39] |
C.L. Sun, H.Q. Peng, L.Y. Niu, et al., Chem. Commun. 54 (2018) 1117-1120. DOI:10.1039/C7CC09315B |
| [40] |
P.Z. Chen, Y.X. Weng, L.Y. Niu, et al., Angew. Chem. Int. Ed. 55 (2016) 2759-2763. DOI:10.1002/anie.201510503 |
| [41] |
H.Q. Peng, Y.Z. Chen, Y. Zhao, et al., Angew. Chem. Int. Ed. 51 (2012) 2088-2092. DOI:10.1002/anie.201107723 |
| [42] |
Z. Xu, S. Peng, Y.Y. Wang, et al., Adv. Mater. 28 (2016) 7666-7671. DOI:10.1002/adma.201601719 |
| [43] |
Q. Zhao, Y. Chen, S.H. Li, et al., Chem. Commun. 54 (2018) 200-203. DOI:10.1039/C7CC08822A |
| [44] |
J.J. Li, Y. Chen, J. Yu, et al., Adv. Mater. 29 (2017) 1701905. DOI:10.1002/adma.201701905 |
| [45] |
D. Zhang, Y. Liu, Y. Fan, et al., Adv. Funct. Mater. 26 (2016) 7652-7661. DOI:10.1002/adfm.v26.42 |
| [46] |
S. Wang, J.H. Ye, Z. Han, et al., RSC Adv. 7 (2017) 36021-36025. DOI:10.1039/C7RA05925F |
| [47] |
D. Jiao, F. Biedermann, F. Tian, et al., J. Am. Chem. Soc. 132 (2010) 15734-15743. DOI:10.1021/ja106716j |
| [48] |
Z.J. Yin, Z.Q. Wu, F. Lin, et al., Chin. Chem. Lett. 28 (2017) 1167-1171. DOI:10.1016/j.cclet.2017.03.029 |
| [49] |
T.T. Cao, X.Y. Yao, J. Zhang, et al., Chin. Chem. Lett. 26 (2015) 867-871. DOI:10.1016/j.cclet.2015.01.032 |
| [50] |
F. Sakai, Z.W. Ji, J.H. Liu, et al., Chin. Chem. Lett. 24 (2013) 568-572. DOI:10.1016/j.cclet.2013.04.027 |
| [51] |
S.H. Li, X. Xu, Y. Zhou, et al., Org. Lett. 19 (2017) 6650-6653. DOI:10.1021/acs.orglett.7b03377 |
| [52] |
X.L. Ni, S. Chen, Y. Yang, et al., J. Am. Chem. Soc. 138 (2016) 6177-6183. DOI:10.1021/jacs.6b01223 |
| [53] |
Y. Xia, S. Chen, X.L. Ni, ACS Appl. Mater. Interfaces 10 (2018) 13048-13052. DOI:10.1021/acsami.8b02573 |
| [54] |
F. Qiao, Z. Yuan, Z. Lian, et al., Dyes Pigm. 146 (2017) 392-397. DOI:10.1016/j.dyepig.2017.07.040 |
| [55] |
H.J. Kim, Paramjyothi C. Nandajan, J. Gierschner, et al., Adv. Funct. Mater. 28 (2017) 1705141. |
 2019, Vol. 30
2019, Vol. 30 

