In addition to the canonical nucleobases of adenine (A), guanine (G), cytosine(C), thymine (T), and uracil (U), many chemical modifications have been identified in both DNA and RNA [1, 2]. These chemical modifications do not change the sequence of DNA and RNA, but alter their structures and biochemical properties, and eventually regulate the spatial and temporal expression of genes [3].
DNA cytosine methylation (5-methylcytosine, 5-mC) is the best-characterized epigenetic modification in genomic DNA [4]. 5-mC modification has been demonstrated to involve in diverse physiological functions [5]. In recent years, increasing numbers of Modifications on genomic DNA have been discovered. Recent studies showed that the ten eleven translocation (TET) proteins were capable of converting 5-mC to generate 5-hydroxymethylcytosine (5-hmC), 5-formylcytosine (5-foC), and finally to 5-carboxylcytosine (5-caC) [6-8]. In addition to DNA cytosine methylation, DNA adenine methylation (N6-methyladenine, m6A) has also been discovered in eukaryotic cells [9, 10]. Similar to 5-mC, these novel modifications identified in DNA have been considered to play critical roles on the regulation of multiple physiological processes as well [11, 12].
RNA is the intermediate molecule that links genetic information from DNA to proteins. Given the important regulatory roles of DNA modification, the recent discovery of reversible modifications on RNA has opened a new era of post-transcriptional gene regulation in eukaryotes [13]. RNA is particularly rich in modifications. Cellular RNA contains more than 150 structurally distinct modifications [14], many of which are considered to be dynamic, reversible and can fine-tune the structures and functions of RNA to influence gene expression [2, 13].
The modifications in DNA and RNA generally have extremely low abundance in vivo [15, 16]. For example, the contents of 5-hmC in both DNA and RNA can be as low as several modifications per million nucleosides [17, 18]. Therefore, sensitive and specific detection methods are essential to dissect the functional roles of these modifications. The developments of new technologies largely revolutionize the epigenetic modification field. Owing to the inherent sensitivity and selectivity, mass spectrometry (MS) has become one of the most prominent analytical techniques [19-21]. However, many DNA and RNA modifications cannot be favorably analyzed by MS, especially for the low abundant modifications.
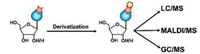
Chemical derivatization has been proved to be a promising strategy to improve the detection performance of analytes in MS [22-24]. Chemical derivatization can alter the chemical and physical properties of analytes. The integration of chemical derivatization with MS analysis can improve the separation and enhance the ionization efficiency during electrospray ionization (ESI)-MS analysis, which eventually lead to the improved detection performance [22]. In the last several years, others and our group have developed various methods for the sensitive and selective analysis of DNA and RNA modifications by chemical derivatization-MS-based strategy (Fig. 1). In this review, we summarized the recent advances for deciphering modifications in nucleic acids by chemical derivatization-MS analysis. Moreover, we discussed their merits and defects and provide typical examples that utilized these techniques to address biological questions.
|
Download:
|
| Fig. 1. The schematic diagram for analysis of DNA and RNA modifications by chemical derivatization-MS-based strategy. Blue circle, red pentagram and yellow pentagon represent nucleobase, modification groups and derivatization reagents, respectively. | |
2. Chemical derivatization-MS-based analysis of nucleic acid modifications
Many DNA and RNA modifications cannot be readily observed by direct MS analysis, especially for the low abundant modifications. In the last decade, the strategy of chemical derivatization in combination with MS analysis has largely improved the detection of modifications in DNA and RNA and promoted the functional studies of these modifications. Since the MS platforms used for the analysis of derivatives are inherently different, we therefore categorized the methods by different MS platforms, i.e., LC/MS, MALDI/MS and GC/MS. We discussed and summarized the advantages and limitations of different methods (Table 1).
|
|
Table 1 Summary of the advantages and limitations of different methods. |
2.1. Chemical derivatization-LC/MS 2.1.1. DNA modifications
5-hmC is now viewed as the sixth base of the genome in mammals besides A, C, T, G, and 5-mC [25]. 5-hmC content in genomic DNA of mammalian cells is low and the quantification of 5-hmC by LC/MS frequently suffers from ion suppression by the presence of high abundant canonical nucleosides. To address the issue, we developed a method by using T4 β-glucosyltransferase to selectively add a glucosyl moiety to the hydroxymethyl group of 5-hmC and form a more hydrophilic residue of β-glucosyl-5-hydroxymethyl-2'-deoxycytidine (5-gmdC), which can be selectively enriched using NH2-silica via hydrophilic interaction followed by LC/MS analysis [26]. Ion suppression from normal nucleosides during MS analysis was then avoided, which contributed to the increased signal intensity of 5-hmC. With the established method, 5-hmC was readily detected in genomes of three human cultured cells as well as seven yeast strains, which was the first report for the existence of 5-hmC in the model organism of yeast.
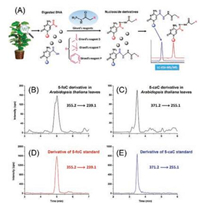
The active DNA demethylation by TET protein-catalyzed oxidation of 5-mC in mammals raised the possible presence of similar demethylation mechanism of 5-mC in plants. To achieve sensitive and quantitative analysis of the relatively low content of 5-foC and 5-caC in DNA of plants, we used Girard's reagent D (GirD), Girard's reagent T (GirT), and Girard's reagent P (GirP) that harbor easily ionized tertiary or quaternary ammonia, to simultaneously react with aldehyde group of 5-foC and carboxyl group of 5-caC under mild conditions (Fig. 2A) [27]. With the established highly sensitive detection method, 5-foC and 5-caC in genomic DNA of Arabidopsis thaliana were distinctly detected (Figs. 2B-E). However, it should be noted that the simultaneous derivatization of 5-foC and 5-caC was performed sequentially, which makes the analytical procedure relatively complicated. Similarly, Hong et al. [28] determined 5-formyl-2'-deoxyuridine (5-foU) by derivatization with GirT that harbors a pre-charged quaternary ammonium moiety. The combination of derivatization with LC/MS analysis could allow for the quantification of 5-foU at a detection limit of 3-4 fmol, which is approximately 20-fold better than that for the direct analysis of native 5-foU.
|
Download:
|
| Fig. 2. (A) The schematic diagram for the determination of 5-foC and 5-caC in genomic DNA of plant samples using chemical derivatization of Girard's reagents coupled with LC/MS analysis. Representative MRM chromatograms for quantification of (B) 5-foC and (C) 5-caC in genomic DNA of Arabidopsis thaliana leaves and derivatization products of (D) 5-foC standard and (E) 5-caC standard. Copied with permission [27]. Copyright 2014, American Chemical Society. | |
5-mC and its oxidation products (5-hmC, 5-foC and 5-caC) differ largely in their abundance in DNA. While direct and simultaneous quantification of these cytosinemodifications is challenging, we developed 2-bromo-1-(4-diethylaminophenyl)-ethanone (BDAPE) derivatization coupled with LC/MS analysis for the sensitive and simultaneous determination of all these four cytosine modifications (5-mC, 5-hmC, 5-foC, and 5-caC) [29]. The derivatization reagent that harbors a hydrophobic phenyl group and an easily chargeable tertiary ammonium group can simultaneously derivatize all the four cytosine modifications. BDAPE readily reacts with the 3-N and 4-N positions of cytosine to form a stable penta cyclic structure. The detection sensitivities of these modifications increased by 35-123 folds after BDAPE derivatization. With this method, we observed the significant depletion of 5-hmC, 5-foC, and 5-caC in human colorectal carcinoma tissues compared to tumor-adjacent normal tissues, suggesting the potential roles of these modifications in the formation of cancers. Since salts can cause the low derivatization efficiency of cytosine modifications by BDAPE, purification of enzymatically digested nucleosides by SPE to remove salts was essential, which however may introduce relatively large deviation.
In addition to BDAPE derivatization, Guo et al. [30] recently employed 4-(dimethylamino) benzoic anhydride to simultaneously derivatize the amino groupof four cytosine modifications (5-mC, 5-hmC, 5-foC, and 5-caC) in DNA. The limits of detection (LODs) ranged from 1.2 fmol to 2.5 fmol. With the established method, they found that the contents of 5-foC and 5-caC increased in human breast cancer tissue compared with tumor-adjacent normal tissue. More recently, Hu et al. [31] developed a strategy of 6-methoxy-2-naphthyl glyoxal hydrate(MTNG) derivatization coupled with online solid-phase extraction (SPE) and LC/MS (SPE-LC/MS) for analysis of 8-nitroguanine, a major mutagenic nucleobase modification generated by peroxynitrite. After derivatization, the sample was analyzed by online SPE-LC/MS and the detection sensitivity increased approximately 10 times. This method was then successfully used to explore the correlation between inflammation-related DNA damage and carcinogenesis. However, MTNG can react with both 8-nitroguanine and other guanine compounds, thus an excess of MTNG was added and SPE was required to remove MTNG prior to MS analysis.
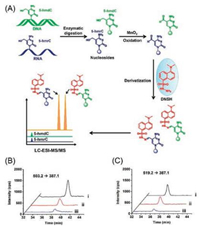
2.1.2. RNA modificationsActive DNA demethylation in mammals can be achieved through oxidation of 5-mC by TET family proteins with the generation of 5-hmC, 5-foC, and 5-caC [11]. TET can also catalyze the formation of 5-hmC from 5-mC in RNA [17]. To explore the possible existence of the further oxidative product of 5-foC from 5-hmC and to achieve simultaneous detection of 5-hmC and 5-foC in RNA, a strategy of oxidation-derivatization combined with MS (ODMS) was developed [32]. In this strategy, MnO2 was utilized to oxide 5-hmC to 5-foC. The aldehyde group in 5-foC can be readily derivatized with the hydrazide moiety in dansylhydrazine (DNSH) to yield hydrazone derivative with an easily chargeable tertiary ammonium, enabling the increased ionization efficiency of 5-foC during LC/MS analysis (Fig. 3A). With this ODMS strategy, we first reported the presence of 5-foC in RNA in mammal cells (Figs. 3B and C). The quantification results showed that 5-foC in RNA were 9.0 ± 1.2/106 rG in HeLa cells and 8.5 ± 1.4/106 rG in 293T cells. The detectable 5-foC in cellular RNA together with the presence of 5-hmC in RNA suggested that the function of TET family proteins can exert epigenetic regulation at both DNA and RNA.
|
Download:
|
| Fig. 3. (A) The schematic diagram for the determination of 5-hmC and 5-foC in both DNA and RNA by oxidation-derivatization coupled with LC/MS analysis. (B) Extracted ion chromatograms of 5-hydroxymethyl-2'-deoxycytosine standard (ⅰ) and 5-hmC detected in DNA of HeLa cells (ⅱ) by ODMS strategy, and endogenous 5-foC detected in DNA of HeLa cells by DNSH derivatization (ⅲ). (C) Extracted ion chromatograms of 5-hydroxymethylcytosine standard (ⅰ) and 5-hmC detected in RNA of HeLa cells (ⅱ) by ODMS strategy, and endogenous 5-foC detected in RNA of HeLa cells by DNSH derivatization (ⅲ). Copied with permission [32]. Copyright 2016, Royal Society of Chemistry. | |
The discovery of 5-hmC and 5-foC in RNA indicated 5-mC in RNA may undergo the same cytosine demethylation pathway with generating 5-hmC, 5-foC, and 5-carboxylcytosine (5-caC) by TET proteins as that in DNA. To explore the existence of 5-caC in RNA, we established 2-bromo-1-(4-diethylaminophenyl)-ethanone (BDEPE) derivatization coupled with LC/MS analysis for sensitive and simultaneous determination of the oxidative products of 5-mC in RNA (Fig. 4A) [33]. The results demonstrated that the detection sensitivities of 5-mC, 5-hmC, 5-foC and 5-caC in RNA increased by 70-313 folds after BDEPE derivatization (Figs. 4B and C). Using this method, we confirmed the existence of 5-caC in RNA of mammals, indicating the possible demethylation pathway of 5-mC in RNA by oxidation with TET proteins. Using the same analytical strategy, we further demonstrated that the levels of 5-hmC, 5-foC, and 5-caC significantly decreased in both the DNA and RNA of mouse embryonic stem cells while exposed to arsenic, cadmium, chromium, and antimony, which suggested a new toxicity mechanism by heavy metals through dysregulating the epigenetic modifications [34].
|
Download:
|
| Fig. 4. (A) Chemical derivatization of the cytosine modifications in RNA. (B) Extracted ion chromatograms of 5-mC, 5-hmC, 5-foC, and 5-caC before (A) and after (B) labeling by BDEPE under optimized conditions. Copied with permission [33]. Copyright 2016, Royal Society of Chemistry. | |
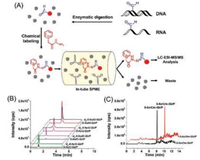
In addition to chemical derivatization-MS analysis, we recently developed a strategy of GirP derivatization combined with in-tube solid-phase microextraction(SPME) and LC/MS (SPME-LC/MS) analysis for the sensitive determination of DNA and RNA formylation (Fig. 5A) [35]. The monolith carrying negative carboxyl group can enrich the positively charged derivatives and eliminate high abundance of normal nucleosides, which further improved the detection performance for analysis of DNA and RNA formylation. Using the method, we were able to simultaneously detect six formylated nucleosides, including 5-foC and 5-formyl-2'-deoxyuridine (5-fodU) from DNA, and 5-foC, 5-foU, 2'-O-methyl-5-formylcytidine (5-foCm) and 2'-O-methyl-5-formyluridine (5-foUm) from RNA of cultured human cells and multiple mammalian tissues (Figs. 5B and C). The detection limits of these formylated nucleosides were improved by 307-884 folds. It was worth noting that 5-foU, 5-foCm and 5-foUm were discovered for the first time in cultured human cells and tissues. This method requires the preparation of monolith carrying negative group, which is tedious and may limit its application in different laboratories.
|
Download:
|
| Fig. 5. (A) The schematic illustration of the analytical procedure by GirP derivatization combined with in-tube SPME-LC/MS analysis for the sensitive determination of DNA and RNA formylation. (B) Extracted-ion chromatograms of GirP-labeled 5-foC and 5-foU from both DNA and RNA of human thyroid carcinoma tissue and d5-GirP-labeled nucleosidestandards. (C) Extracted-ion chromatograms of GirP-labeled 5-forCm and 5-forUm from RNA of human thyroid carcinoma tissue. Copied with permission [35]. Copyright 2017, Elsevier. | |
Along with ESI-MS, the utility of inductively coupled plasma mass spectrometry(ICP-MS) is an alternative and complementary tool in study of nucleic acidmodifications. Wrobel et al. [36] proposed a strategy enabling sensitive detection of 5-mC in RNA based on LC-ICP-MS detection. The strategy relies on derivatization of ribose with osmium (Os) by formation of a ternary complex between cis-diol ribose groups, K2OsO2(OH)4 and tetramethylethylenediamine. The obtained detection limit of 5-mC was 21 pmol/L, demonstrating a sensitive quantification of 5-mC.
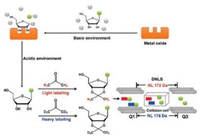
2.1.3. Free nucleoside/nucleotide modificationsDetection of endogenous modified nucleosides in biological fluids may serve as a non-invasive manner for diseases diagnostics. We developed a strategy for comprehensive profiling of modified nucleosides from biological fluids using metal oxide-based dispersive solid-phase extraction (DSPE) followed with stable isotope labeling and double neutral loss scan-mass spectrometry analysis (Fig. 6) [37]. Cerium dioxide (CeO2) was used to selectively capture ribose conjugates from complex biological samples under basic environment. The enriched nucleosides were then derivatized with acetone and acetone-d6. The acetone and acetone-d6 derivatized compounds were ionized at the same condition but recorded separately on MS spectra, which can significantly improve the detection specificity and promote the identification of modified nucleosides. Using the developed method, we profiled the modified nucleosides in human urine and 49 ribose conjugates were readily identified, among which 7 ribose conjugates exhibited significant contents change between healthy controls and lymphoma patients. Later, Li et al. [38] used the similar strategy to identify 52 modified nucleosides in urine sample. Since this analytical strategy depends on the enrichment of nucleosides that carry the cis-diol ribose, the 2'-O-methylation nucleosides cannot be captured and detected.
|
Download:
|
| Fig. 6. General procedure for the comprehensive profiling of modified nucleosides from biological fluids using metal oxide-based dispersive solid-phase extraction followed with stable isotope labeling and double neutral loss scan-mass spectrometry analysis. Copied with permission [37]. Copyright 2015, American Chemical Society. | |
In addition to methyltransferase-mediated DNA and RNA methylation, premethylated nucleotides can be potentially incorporated into DNA and RNA during replication and transcription. To explore the possible existence of endogenous modified nucleotides, we established a method by N, N-dimethyl-p-phenylenediamine (DMPA) derivatization coupled with LC/MS for sensitive and simultaneous determination of 10 nucleotides, including 5-methyl-2'-deoxycytidine monophosphate (5-Me-dCMP) and 5-methylcytidine monophosphate (5-Me-CMP) (Fig. 7A) [39]. After DMPA derivatization, the detection sensitivities of nucleotides increased by 88-372 folds, which can be attributed to the introduction of a tertiary ammonia group and a hydrophobic moiety from DMPA. Using this method, we found that endogenous 5-Me-dCMP and 5-Me-CMP widely existed in cultured human cells, human tissues, and human urinary samples (Fig. 7B). This study is the first report for detection of endogenous 5-Me-dCMP and 5-Me-CMP in mammals. Very recently, we further established 8-(diazomethyl)quinoline derivatization in conjugation with LC/MS analysis for determination of endogenous modified nucleoside triphosphates (NTPs) in the mammalian cells and tissues [40]. The synthesized 8-(diazomethyl)quinoline could efficiently react with the phosphate group of NTPs under mild condition. The developed method allowed the sensitive detection of NTPs with the detection limits improved by 56-137 folds. With this method, 12 types of endogenous modified NTPs were distinctly determined in the mammalian cells and tissues. These studies revealed the widespread existence of various modified NTPs in eukaryotes, which may provide new source for modifications of DNA and RNA. The 8-(diazomethyl)quinoline is less stable since the diazo group is easy to degrade, which requires extra care while performing the derivatization reaction.

|
Download:
|
| Fig. 7. (A) Chemical labeling of nucleotides by DMPA. "B" in nucleotides represents nucleobase. (B) Extracted-ion chromatograms of DMPA-d4-labeled 5-Me-dCMP and 5-Me-CMP standards, DMPA-labeled 5-Me-dCMP and 5-Me-CMP from the human urine, and 293T cells and HeLa cells. Copied with permission [39]. Copyright 2017, American Chemical Society. | |
2.2. Chemical derivatization-MALDI/MS
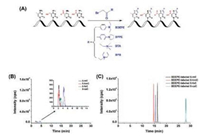
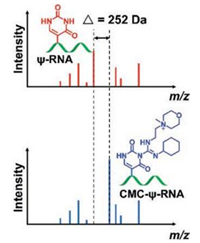
Pseudouridine (Ψ), the isomerized form of uridine with C5 and N1 position interconversion, is a mass-silent modification in RNA, which results in the inability of normal MS to identify Ψ. To address the issue, Patteson et al. [41] developed a method of 1-cyclohexyl-3-(2-morpholinoethyl) carbodiimide (CMC) derivatization combined with matrix-assisted laser desorption/ionization-MS (MALDI/MS) analysis for the determination of Ψ in RNA. After CMC derivatization, each Ψ exhibited a mass increase of 252 Da, which allows for easy characterization of Ψ by MALDI/MS (Fig. 8). To determine the sequence location of Ψ, MALDI/MS analysis of RNase T1 digestion products before and after CMC derivatization was performed. However, the CMC was also found to modify other uridine nucleosides, which potentially can be problematic when analyzing RNAs that have many uridine residues. Later, the same group improved the CMC derivatization conditions by optimizing the reaction time, temperature, pH and the ratio of CMC amount to sample [42]. Under the optimized derivatization conditions, false positive by incomplete derivatization can be minimized. This approach can provide information for specific small RNAs that contain Ψ [43].
|
Download:
|
| Fig. 8. The schematic illustration of CMC derivatization combined with MALDI/MS analysis for the determination of Ψ residues in RNA. After CMC derivatization, each Ψ residue exhibits a mass increase of 252 Da, which allows for easy characterization of Ψ by MALDI/MS. | |
Specific cyanoethylation of N1 in Ψ has been known for decades [44]. Compared to CMC derivatization, Ψ can be completely cyanoethylated while normal uridine is only ~5% modified, enabling a single-step derivatization process without the effect of destroy alkali-labile backbone of RNA. Using this derivatization strategy, Mengel-Jùrgensen et al. [45] employed acrylonitrile to derivatize Ψ in tRNA. Cyanoethylation of Ψ led to a 53 Da mass increase, which can be easily characterized in MALDI/MS analysis. With this strategy, one Ψ in tRNATyrII from E. coli was successfully identified. MALDI/MS in combination with cyanoethylation has been demonstrated to be a useful complement to the CMC derivatization with MS detection.
2.3. Chemical derivatization-GC/MSGas chromatography-MS (GC/MS) has been widely used in the determination of trace amounts of compounds [46, 47]. However, DNA and RNA modifications cannot be directly analyzed by GC/MS since these compounds are not volatile. Therefore, chemical derivatization is typically used to convert polar nucleobases/nucleosides into volatile derivatives before GC/MS analysis.
Singer et al. [48] developed a method for the detection of 5-mC in genomic DNA of calf thymus, salmon sperm and several mouse tissues. DNA was hydrolyzed with 88% formic acid at 180 ℃ into nucleobases followed by bis(trimethylsilyl)trifluoroacetamide (BSTFA) derivatization. The resulting derivatives of dC and 5-mC can be easily separated without interference of dA, dG and T. As little as 1.6 pmol of 5-mC in DNA can be detected. Later, Romerio et al. [49] introduced two isotopically labelled internal standards, [2-13C]5-methylcytosine and [2-13C]cytosine, which provided more accurate quantification of 5-mC in genomic DNA. The method was successfully applied to the detection of 5-mC in DNA of peripheral blood mononuclear cell.
In 2012, our group developed a highly sensitive and reliable method for the systematic study on the existence of 5-mC in yeast genomes by GC/MS [50]. The extracted DNA was hydrolyzed to purines and pyrimidines with 88% aqueous formic acid, followed by derivatization with BSTFA and cholorotrimethylsilane in acetonitrile. The results showed that the LOD of 5-mC was 0.8 pg (6.4 fmol). Using this developed method, we found that 5-mC was present in 19 yeast strains within the range of 0.014%-0.364%, indicating a widespread DNA methylation in yeast genomes.
3. Conclusions and perspectivesThe recent advances demonstrate a dynamic view of DNA and RNA modifications, which increase the diversity of nucleic acids, and more importantly, add additional layers to the regulation of physiological processes. Investigations of the functions of DNA and RNA modifications provide valuable insights into the understanding of the molecular mechanism of diseases. Continued and rapid improvements in techniques make the study of DNA and RNA modifications more accessible. In this review, we focused on the chemical derivatization-MS based analytical strategy for deciphering DNA and RNA modifications.
Design and use of proper derivatization reagents to achieve fast, efficient and specific labeling are important for the study of nucleic acid modifications by chemical derivatization-MS analysis in the future. However, there are still many challenges to the effective determination of DNA and RNA modifications. It should be noted that the limitations of derivatization-based MS include by-product formation and interference caused by excess derivatization reagents. Therefore, the development of new derivatization regents and reactions are still desired for the sensitive and selective detection of diverse nucleic acid modifications by MS. Analysis of the chemical structures of DNA and RNA modifications will provide useful guide to prepare appropriate derivatization reagents. The development of new derivatization reactions may lead to the discovery of novel DNA and RNA modifications that will provide valuable clues underlying association of nucleic acid modifications with diseases.
In addition to decipher the modifications in DNA and RNA, chemical derivatization in combination with MS analysis can also be expanded to various research fields, such as proteomics study, metabolomics study, and MS imaging.
AcknowledgmentsThe work is supported by the National Key R & D Program of China (No. 2017YFC0906800), and the National Natural Science Foundation of China (Nos. 21522507, 21672166, 21635006 and 21721005).
| [1] |
K. Chen, B.S. Zhao, C. He, Cell Chem. Biol. 23 (2016) 74-85. DOI:10.1016/j.chembiol.2015.11.007 |
| [2] |
T. Liu, C.J. Ma, B.F. Yuan, Y.Q. Feng, Sci. China Chem. 61 (2018) 381-392. DOI:10.1007/s11426-017-9186-y |
| [3] |
Y. Fu, D. Dominissini, G. Rechavi, C. He, Nat. Rev. Genet. 15 (2014) 293-306. DOI:10.1038/nrg3724 |
| [4] |
S. Feng, S.E. Jacobsen, W. Reik, Science 330 (2010) 622-627. DOI:10.1126/science.1190614 |
| [5] |
Z.D. Smith, A. Meissner, Nat. Rev. Genet. 14 (2013) 204-220. |
| [6] |
S. Kriaucionis, N. Heintz, Science 324 (2009) 929-930. DOI:10.1126/science.1169786 |
| [7] |
M. Tahiliani, K.P. Koh, Y. Shen, et al., Science 324 (2009) 930-935. DOI:10.1126/science.1170116 |
| [8] |
S. Ito, L. Shen, Q. Dai, et al., Science 333 (2011) 1300-1303. DOI:10.1126/science.1210597 |
| [9] |
G.Z. Luo, M.A. Blanco, E.L. Greer, C. He, Y. Shi, Nat. Rev. Mol. Cell Biol. 16 (2015) 705-710. |
| [10] |
W. Huang, J. Xiong, Y. Yang, et al., RSC Adv. 5 (2015) 64046-64054. DOI:10.1039/C5RA05307B |
| [11] |
X. Wu, Y. Zhang, Nat. Rev. Genet. 18 (2017) 517-534. |
| [12] |
G.Z. Luo, C. He, Nat. Struct. Mol. Biol. 24 (2017) 503-506. DOI:10.1038/nsmb.3412 |
| [13] |
I.A. Roundtree, M.E. Evans, T. Pan, C. He, Cell 169 (2017) 1187-1200. DOI:10.1016/j.cell.2017.05.045 |
| [14] |
P. Boccaletto, M.A. Machnicka, E. Purta, et al., Nucleic Acids Res. 46 (2018) D303-D307. DOI:10.1093/nar/gkx1030 |
| [15] |
B.F. Yuan, Y.Q. Feng, TrAC-Trend. Anal. Chem. 54 (2014) 24-35. DOI:10.1016/j.trac.2013.11.002 |
| [16] |
B.F. Yuan, Adv. Clin. Chem. 67 (2014) 151-187. DOI:10.1016/bs.acc.2014.09.003 |
| [17] |
L. Fu, C.R. Guerrero, N. Zhong, et al., J. Am. Chem. Soc. 136 (2014) 11582-11585. DOI:10.1021/ja505305z |
| [18] |
S. Liu, J. Wang, Y. Su, et al., Nucleic Acids Res. 41 (2013) 6421-6429. DOI:10.1093/nar/gkt360 |
| [19] |
M. Kandiah, P.L. Urban, Chem. Soc. Rev. 42 (2013) 5299-5322. DOI:10.1039/c3cs35389c |
| [20] |
Z.J. Wang, L.L. Chi, Chin. Chem. Lett. 29 (2018) 11-18. DOI:10.1016/j.cclet.2017.08.050 |
| [21] |
H.Z. Zhao, J.F. Li, X.L. Ma, et al., Chin. Chem. Lett. 29 (2018) 102-106. DOI:10.1016/j.cclet.2017.06.013 |
| [22] |
B.L. Qi, P. Liu, Q.Y. Wang, et al., TrAC-Trend. Anal. Chem. 59 (2014) 121-132. DOI:10.1016/j.trac.2014.03.013 |
| [23] |
Q.Y. Cheng, J. Xiong, F. Wang, et al., Chin. Chem. Lett. 29 (2018) 115-118. DOI:10.1016/j.cclet.2017.06.009 |
| [24] |
B.F. Yuan, Q.F. Zhu, N. Guo, et al., Anal. Chem. 90 (2018) 3512-3520. DOI:10.1021/acs.analchem.7b05355 |
| [25] |
M. Munzel, D. Globisch, T. Carell, Angew. Chem. Int. Ed. Engl. 50 (2011) 6460-6468. DOI:10.1002/anie.v50.29 |
| [26] |
Y. Tang, J.M. Chu, W. Huang, et al., Anal. Chem. 85 (2013) 6129-6135. DOI:10.1021/ac4010869 |
| [27] |
Y. Tang, J. Xiong, H.P. Jiang, et al., Anal. Chem. 86 (2014) 7764-7772. DOI:10.1021/ac5016886 |
| [28] |
H. Hong, Y. Wang, Anal. Chem. 79 (2007) 322-326. DOI:10.1021/ac061465w |
| [29] |
Y. Tang, S.J. Zheng, C.B. Qi, Y.Q. Feng, B.F. Yuan, Anal. Chem. 87 (2015) 3445-3452. DOI:10.1021/ac504786r |
| [30] |
M. Guo, X. Li, L. Zhang, et al., Oncotarget 8 (2017) 91248-91257. |
| [31] |
C.W. Hu, Y.J. Chang, J.L. Chen, Y.W. Hsu, M.R. Chao, Molecules 23 (2018) 605. DOI:10.3390/molecules23030605 |
| [32] |
H.Y. Zhang, J. Xiong, B.L. Qi, Y.Q. Feng, B.F. Yuan, Chem. Commun. 52 (2016) 737-740. DOI:10.1039/C5CC07354E |
| [33] |
W. Huang, M.D. Lan, C.B. Qi, et al., Chem. Sci. 7 (2016) 5495-5502. DOI:10.1039/C6SC01589A |
| [34] |
J. Xiong, X. Liu, Q.Y. Cheng, et al., ACS Chem. Biol. 12 (2017) 1636-1643. DOI:10.1021/acschembio.7b00170 |
| [35] |
H.P. Jiang, T. Liu, N. Guo, et al., Anal. Chim. Acta 981 (2017) 1-10. DOI:10.1016/j.aca.2017.06.009 |
| [36] |
K. Wrobel, C. Rodriguez Flores, Q. Chan, K. Wrobel, Metallomics 2 (2010) 140-146. DOI:10.1039/B915474D |
| [37] |
J.M. Chu, C.B. Qi, Y.Q. Huang, et al., Anal. Chem. 87 (2015) 7364-7372. DOI:10.1021/acs.analchem.5b01614 |
| [38] |
S. Li, Y. Jin, Z. Tang, et al., Anal. Chim. Acta 864 (2015) 30-38. DOI:10.1016/j.aca.2015.01.044 |
| [39] |
H. Zeng, C.B. Qi, T. Liu, et al., Anal. Chem. 89 (2017) 4153-4160. DOI:10.1021/acs.analchem.7b00052 |
| [40] |
H.P. Jiang, J. Xiong, F.L. Liu, et al., Chem. Sci. 9 (2018) 4160-4167. DOI:10.1039/C7SC05472F |
| [41] |
K.G. Patteson, L.P. Rodicio, P.A. Limbach, Nucleic Acids Res. 29 (2001) E49-49. DOI:10.1093/nar/29.10.e49 |
| [42] |
A. Durairaj, P.A. Limbach, Anal. Chim. Acta 612 (2008) 173-181. DOI:10.1016/j.aca.2008.02.026 |
| [43] |
A. Durairaj, P.A. Limbach, Rapid Commun. Mass Spectrom. 22 (2008) 3727-3734. DOI:10.1002/rcm.v22:23 |
| [44] |
M. Yoshida, T. Ukita, Biochim. Biophys. Acta 157 (1968) 466-475. DOI:10.1016/0005-2787(68)90146-9 |
| [45] |
J. Mengel-Jorgensen, F. Kirpekar, Nucleic Acids Res. 30 (2002) e135. DOI:10.1093/nar/gnf135 |
| [46] |
N. Krone, B.A. Hughes, G.G. Lavery, et al., J. Steroid Biochem. Mol. Biol. 121 (2010) 496-504. DOI:10.1016/j.jsbmb.2010.04.010 |
| [47] |
F. Wang, A. Huang, X. Yin, W. Wang, J. Chen, Chin. Chem. Lett. 29 (2018) 1395-1398. DOI:10.1016/j.cclet.2017.11.007 |
| [48] |
J. Singer, W.C. Schnute Jr, J.E. Shively, C.W. Todd, A.D. Riggs, Anal. Biochem. 94 (1979) 297-301. DOI:10.1016/0003-2697(79)90363-4 |
| [49] |
A.S. Romerio, G. Fiorillo, I. Terruzzi, et al., Anal. Biochem. 336 (2005) 158-163. DOI:10.1016/j.ab.2004.09.034 |
| [50] |
Y. Tang, X.D. Gao, Y. Wang, B.F. Yuan, Y.Q. Feng, Anal. Chem. 84 (2012) 7249-7255. DOI:10.1021/ac301727c |
 2019, Vol. 30
2019, Vol. 30 


