b Department of Applied Physics, South China University of Technology, Guangzhou 510641, China;
c State Key Laboratory of Optoelectronics Materials and Technologies, Sun Yat-sen University, Guangzhou 510275, China;
d Department of Chemistry, Michigan State University, E. Lansing, MI 48824, United States
Corrole is an analogue of porphyrin lacking one meso carbon atom, which have recently been widely studied due to their extensive applications in photodynamic detection [1], dye-sensitized solar cells [2], optical imaging [3], photodynamic therapy [4-6], photodynamic inactivation [7], catalysis [8], and as drug candidates for diseases characterized by oxidative stress [9-11]. The much interests in corrole have been facilitated with the development of its simple synthetic procedure [12].
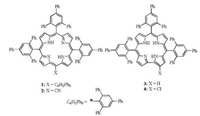
Halogenation of β-pyrrole carbon atoms is an important way in modifying corrole. It has significant effect on spectroscopic, electrochemical and catalytic properties of corroles [13-16]. The absorption spectra of halogenated corroles display bathochromic shift and the redox potentials move to much more positive potentials as compared to the non-halogenated counterparts [17, 18]. Iodination of gallium or aluminum corrole has significant effect on their photophysical and electrochemical properties [18]. The fully octa-β-chlorinated phosphorus and gallium corroles may be used in photoelectrochemical cell [17]. Furthermore, postfunctionalization is often performed by using more stable metallocorroles instead of their free base counterparts to avoid oxidative degradation [19-21]. Although, the fully octa-β-chlorinated free base corrole had been prepared by reductive demetallation of β-chlorinated copper corrole [22], the regioselective halogenation of corrole remains considerable challenge yet [23-27]. The lacking of one meso carbon atom decreases the symmetry of the corrole ring, as a result, the peripheral functionalization can theoretically leads to a significant number of different regioisomers [28]. However, the directly linked pyrrole β-H of corrole demonstrates more reactive, this makes regioselectivity possible during the peripheral functionalization, which usually undergoes on positions 2 (18) or 3 (17) [29, 30]. Recently, Nardis and his coworkers had reported the regioselective synthesis of mono halogenated corrole 2-Br-TTCorrH3, 3-Br-TTCorrH3, 3-Cl-TTCorrH3 and di-halogenated 3,17-Cl2-TTCorrH3 using halo acids as halogenating agent [29]. Generally, poly-pyrrolic macrocycle might be prepared by acid catalyzed one-pot condensation of pyrrole and aldehyde followed by oxidation with DDQ. A4-type porphyrin [31], A3-type porphyrin [32], N-confused porphyrin [31], expanded porphyrin [33] and corrole [34] can be prepared by controlling the reaction conditions. Recently, we have identified A3B-type meso-cyano porphyrin 2 (H2TTPPPCN, Fig. 1) [35], in which DDQ served as oxidant and cyanating agent. Herein, we wish to report the case synthesis of 2-chloro bulky bis-pocket corrole 4 (H3TTPPC-Cl, Fig. 1), its crystallographic characterization, and photophysical properties. This mono chlorinated corrole was obtained as a by-product during the synthesis of its mother bulky bis-pocket corrole 3 (H3TTPPC) [36], in which DDQ served as oxidant and chlorinating agent.
|
Download:
|
| Fig. 1. Molecular structures of porphyrins and corroles. | |
Metal porphyrinoids with peculiar electronic or steric properties are excellent mechanistic probes for the oxygenation reaction [13, 37, 38]. Employment of sterically crowded porphyrin may avoid the formation of μ-oxo and μ-peroxo dimer intermediates during the catalytic reaction [23]. This approach was maneuvered in synthesis of stericallycrowded bis-pocketed porphyrin, 5,10,15,20-tetrakis(2,4,6-triphenylphenyl)-porphyrin 1 (H2TTPPP) [39], and corrole, 5,10,15-tris(2,4,6-triphenylphenyl)-corrole 3 (H3TTPPC) [36] that has non-polar pockets on both faces of the macrocycle.
In 1983, Suslick reported for the first time the synthesis of bispocket 5,10,15,20-tetrakis(2,4,6-triphenylphenyl)-porphyrin 1 (H2TTPPP) in 1% yield by slow addition of a stoichiometric amount of pyrrole diluted in xylenes to a refluxing propionic acid solution of 2,4,6-triphenyl-benzaldehyde [39]. It can also be prepared by condensation of pyrrole and 2,4,6-triphenyl-benzaldehyde using 2,4,6-collidine as solvent at elevated temperature [40]. Lindsey [41, 42] and Drenth [43] independently observed that strong acid catalyzed condensation of pyrrole and aldehyde under inert atmosphere in chlorinated solvents (CH2Cl2 or CHCl3) may significantly improve the yield of porphyrins with bulky substituents at the ortho positions. Previously, we have observed that the trifluoroacetic acid (TFA) may intensely increase the yield of corrole 3 (H3TTPPC) [36] in the catalytic condensation of 2,4,6-triphenyl-benzaldehyde and its dipyrromethane. However, we found that the employment of TFA in the direct condensation of same aldehyde and pyrrole only afforded an A3B-type meso-cyano porphyrin 2 (H2TTPPPCN) with a yield of 4% [35], and no bis-pocket porphyrin 1 could be isolated. Is it possible to prepare bulky porphyrin 1 by TFA catalyzed condensation of 2,4,6-triphenylbenzaldehyde and its dipyrromethane? A careful investigation leads to current finding of a newlight green color 2-chloro-5,10,15-tris(2,4,6-triphenylphenyl)-corrole 4 (H3TTPPC-Cl) (Scheme 1).

|
Download:
|
| Scheme 1. Syntheses of 3 (H3TTPPC) and 4 (H3TTPPC-Cl). | |
We performed the synthetic experiment by one-pot two steps procedure as described in Supporting information.Except for normal corrole 3(H3TTPPC, yield3.9%), a new green compound 4 with close Rf value to 3 was obtained with an isolated yield of 5.7%. UV–vis spectrum of 4 indicated that it was a porphyrinoid macrocycle. Surprisingly, ESI-HR mass spectrum of 4 showed mass ion peak at 1245.4658 (Fig. S2 in Supporting information) which was neither corresponding to A4-type porphyrin 1 (H2TTPPP) nor to corrole 3 (H3TTPPC), even not to A3B-type meso-cyano porphyrin 2 (H2TTPPPCN). The difference of 281 mass units between the observed mass and A4-type porphyrin 1 (H2TTPPP) vanished the possibility of formation of A4-type porphyrin 1 (H2TTPPP). Nonetheless, difference of 3 mass units eradicated the possibility of formation of A3B-type meso-cyano porphyrin 2 (H2TTPPPCN). Nevertheless, it even eliminated the chance of formation of A3-type porphyrin because the difference of 23 mass units between the observed mass.1HNMR spectra provide decisive information about the macrocycle; whether it is porphyrin or not may be easily determined by the presence of high field proton [35]. The 1H NMR spectrum of 4 revealed the absence of any high field proton eradicating the formation of a porphyrin derivative (Fig. S4 in Supporting information). The 1H NMR spectrum of unexpected macrocycle 4 showed the same number of phenyl proton with corrole 3(H3TTPPC)(Figs. S3 and S4 in Supporting information), but one less β-proton was observed in its 1H NMR spectrum (Fig. 2). From these observations, we hypothesized that the unexpected product was a mono-β-chlorinated derivative of its parent corrole 3 (H3TTPPC), and this was strongly supported by its HR-MS spectrum.

|
Download:
|
| Fig. 2. 1H NMR spectra of the β-pyrrole protons region of 3 (H3TTPPC) (above) and 4 (H3TTPPC-Cl) (down). | |
We have succeeded to get suitable single crystal of 4 (H3TTPPC-Cl) by slow evaporation from a solvent system of dichloromethane/methanol for crystallographic characterization. Its CCDC number is 1584077. The crystal structure revealed that one β-proton on position 2 was substituted by chloride in the unexpected 2-chloro-5,10,15-tris(2,4,6-triphenylphenyl)-corrole 4 (Scheme 2), and the crystal had a formulation unit in the cell with a triclinic P-1 space group. Single crystal X-ray structure also revealed that macrocycle 4 had a distorted planar structure. The bond length between C2-Cl1(2) is 1.700Å. The bond angle between C11-N3-C14, C16-N2-C19, C1-N1-C4 and C6-N4-C9 is 109.49(16)°, 109.73(17)°, 111.70(16)° and 109.38(17)° respectively with average value of 110.07°. The average distance between 23 core atoms of corrole is 1.395Å (Table S1 in Supporting information). Interestingly, crystal-packing of 4 (H3TTPPC-Cl) showed a one dimensional chain structure, making hydrogen bonding with hydrogen atom of triphenylbenzene and the chlorine present on position 2 of corrole skeleton (C—H…Cl). Corrole molecules have interplanar distance of 9.26Å approximately, in which neighboring corrole molecules take 180° off-set arrangement in regular manner (Scheme S1 in Supporting information).

|
Download:
|
| Scheme 2. ORTEP plots of 4 (H3TTPPC-Cl) (Showing 50% probability thermal displacement ellipsoids, hydrogen atoms are omitted for clarity). | |
Recently, Nardis and coworker have reported the regioselective chlorination of 5,10,15-tritolylcorrole using DDQ as oxidant and hydrochloric acid as chlorination source through radical cation [29]. They had tested many haloacids and found that the halogenation regioselectivity depended on the size of halogen atom and steric hindrance produced by meso substituent. In our case, no haloacids was present in the reaction system. Thus, the chloride in 4 might come from DDQ. A possible mechanism for the formation of mono chlorinated bulky bis-pocket corrole 4 was depicted in Scheme 3. In which the DDQ caused the oxidation of corrole to produce radical cation intermediate, followed by the substitution of hydrogen by chloride atom from DDQ. Steric crowding provided by the bulky groups at meso positions may facilitate the chlorination on position 2. The support of this mechanism comes from the recent observation of ortho-chlorination of 2-arylpyridines by DDQ [44]. The other possible way is that the chloride comes from chlorinated solvent, dichloromethane. Detailed mechanism of this reaction still needs further investigation. Halogen substituted corrole is considered as one of the most efficient precursor that can be further modified through Pdcatalyzed cross-coupling reactions, such as the Suzuki, Heck, Sonogashira, Stille, Negishi or Kumada procedures, where the Chalogen bond can be easily replaced with a stable C—C bond, and this approach has been well maneuvered for the preparation of undecaryl-corroles [45], tetrabenzo-corroles [46-49] and alkynylcorroles [50]. Our case finding in the preparation of selective chlorinated corrole may have practical uses in the synthesis of more sophisticated corrole architectures, having specific features for versatile applications.

|
Download:
|
| Scheme 3. Proposed pathway for chlorination. | |
With the new mono chlorinated bis-pocket corrole 4 in hand, we have investigated the chlorination effect on its photophysical and electrochemical properties. Fig. 3 shows the UV–vis spectra of corroles 3 (H3TTPPC) and 4 (H3TTPPC-Cl) in dichloromethane and toluene respectively. Both the corroles displayed typical soret band and Q-bands. It has been reportedthat theaddition of chlorine atom at the β-position of corrole will induce a red shift in their UV–vis spectra [17, 29, 51]. Here, we observed that the substitution of chloride onposition 2 induced the slight red shift in Soretband with an average of 1–2 nm, but in the terminal Q band a larger red shift of average 3 nm was observed (Table S2 in Supporting information). DFT calculated electronic spectra of 3(H3TTPPC)and 4(H3TTPPC-Cl) also demonstrated this chlorination induced red shift effect (Fig. S5 in Supporting information). The simulated Soret band and Q-band were found to be red shifted by 4.9 nm and 12.5 nm, respectively. The larger shift for Q-band is consistent with experimental results.

|
Download:
|
| Fig. 3. UV–vis spectra of 3 (H3TTPPC) (black line) and 4 (H3TTPPC-Cl) (red line) in dichloromethane (a) and toluene (b). | |
Chlorination has a significant effect on the excited states characteristics of corroles. Previously, our group has reported that the substitution of one chloride at one of the remote ring of triarlycorroles significantly decreases its fluorescence quantum yield due to increase in the intersystem crossing rate constant [15]. Significant decrease in fluorescence intensity for the β-pyrrole chlorinated corrole 4 (H3TTPPC-Cl) was also observed, and the fluorescence emission maxima was red shifted as compared to non-chlorinated corrole (Figs. S6a and b in Supporting information). Furthermore, the fluorescence decay were measured by exciting the samples at 440 nm (Fig. 4). The obtained decay profile for corroles complied with single exponential fitting, indicating one luminescent component in both cases. The calculated fluorescence lifetimes are 4.2 ns and 2.5 ns for 3 (H3TTPPC) and 4 (H3TTPPC-Cl), respectively. Fluorescence quantum yields were measured with excitation at 550 nm, using tetra-phenylporphyrin (H2TPP) as reference (Φf = 0.11) [52], the data demonstrated lower fluorescence quantum yield of chlorinated corrole as compared to non-chlorinated corrole (Table S3 in Supporting information).

|
Download:
|
| Fig. 4. Fluorescence decay curve of 3 (H3TTPPC) (black line) and 4 (H3TTPPC-Cl) (blue line) in dichloromethane. | |
Cyclic voltammetry was used to investigate the effect of chlorination on the redox potential. We were observed three redox events for both corroles: one reversible oxidation and two irreversible reductions (Fig. 5). Generally, halogenation of the corrole at β-pyrrole position shifted the redox potential towards more positive potential [17, 18]. In case of 3 (H3TTPPC), the reversible oxidation took place at E1/2 = -0.05 V (vs. Ag/AgNO3), while for 4 (H3TTPPC-Cl) the reversible oxidation took place at E1/2 = 0.04 V (vs. Ag/AgNO3) with 90 mV shift in the oxidation potential (Table S4 in Supporting information).

|
Download:
|
| Fig. 5. Cyclic voltammograms of corrole 3 (H3TTPPC) (black line) and 4 (H3TTPPC-Cl) (red line) (2.4 μmol/L) in 0.1 mol/L [n-Bu4N]ClO4 DMF solution at a glassy carbon electrode with the scan rate of 100 mV/s. | |
Lastly, We have used the 1,3-diphenylisobenzofuran (DPBF) to investigate the singlet oxygen generation ability of corrole 3 (H3TTPPC) and 4 (H3TTPPC-Cl) under irradiation. When the DPBF reacts with singlet oxygen, the intensity of absorption band at 418 nm decreases (Fig. S7 in Supporting information). The absorption intensity of DPBF decreased with the increasing illumination time (0–9 min) in the presence of corroles. We may evaluate the relative ability of the 3 (H3TTPPC) and 4 (H3TTPPC-Cl) in generating singlet oxygen from slope of the line. The slope of line was increased in the presence of 3 (H3TTPPC) and 4 (H3TTPPC-Cl), indicating these corroles may generating singlet oxygen after irradiation (Fig. 6). From the slope of line it was observed that the singlet oxygen generation ability of 3 (H3TTPPC) was higher than that of 4 (H3TTPPC-Cl). Normally, the singlet oxygen generation depends on the population of triplet state which transfers energy to ground state oxygen (triplet state) [53]. However, the singlet oxygen generation depend not only on the intersystem crossing but also on the efficiencyof energy transfer from triplet state of a photosensitizer to ground state of oxygen [14]. The reduction in singlet oxygen generation in case of 4 (H3TTPPC-Cl) can be attributed to the inefficient quenching of triplet state by oxygen [54].

|
Download:
|
| Fig. 6. Plot of change in absorbance of DPBF (blue line) at 418 nm vs. irradiation time in the presence of 3 (H3TTPPC) (black line) and 4 (H3TTPPC-Cl) (red line) in DMF. | |
In conclusion, we have observed the selective chlorination of bulky bis-pocket corrole on the position 2 of the corrole macrocycle during synthesis of its parent corrole, in which DDQ serves as oxidant and chlorination reagent. 1H NMR, UV–vis, high resolution mass spectroscopy and X-ray crystallography have been used for characterization. The absorption and emission spectra display red shift, with decrease in fluorescence intensity and decrease in the photo quantum yield of chlorinated corrole.Thefluorescence lifetime decrease may be due to increase in intersystem crossing. This indicates that β-chlorination has significant effect on its photophysical properties. Electrochemical investigation reveals positive shift of redox potentials for chlorinated corrole. Furthermore, we have observed that the singlet oxygen generation by non-chlorinated corrole was higheras compared to chlorinated corrole, which is probablycaused by the inefficient quenching of triplet state by oxygen.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21671068, 81400023, 61178037, 81273549), National Basic Research Program (973 Program) of China (No. 2013CB922403) and the Open Fund of State Key Laboratory of Optoelectronic Materials and Technologies, Sun Yatsen University (No. OEMT-2015-KF-05).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.03.006.
| [1] |
H. Agadjanian, J. Ma, A. Rentsendorj, et al., Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 6105-6110. DOI:10.1073/pnas.0901531106 |
| [2] |
D. Walker, S. Chappel, A. Mahammed, et al., J. Porphyrins Phthalocyanines 10 (2006) 1259-1262. DOI:10.1142/S1088424606000624 |
| [3] |
J.Y. Hwang, S.W. Hogiu, V.K. Ramanujan, et al., Biomed. Opt. Express 2 (2011) 356-364. DOI:10.1364/BOE.2.000356 |
| [4] |
F. Cheng, L. Huang, H. Wang, et al., Chin. J. Chem. 35 (2017) 86-92. DOI:10.1002/cjoc.v35.1 |
| [5] |
Z. Zhang, J.Y. Wen, B.B. Lv, et al., Appl. Organomet. Chem. 30 (2016) 132-139. DOI:10.1002/aoc.v30.3 |
| [6] |
Z. Zhang, H.H. Wang, H.J. Yu, et al., Dalton Trans. 46 (2017) 9481-9490. DOI:10.1039/C7DT00992E |
| [7] |
A. Preuß, I. Saltsman, A. Mahammed, et al., J. Photochem. Photobiol. B 133 (2014) 39-46. DOI:10.1016/j.jphotobiol.2014.02.013 |
| [8] |
I. Luobeznova, M. Raizman, I. Goldberg, Z. Gross, Inorg. Chem. 45 (2006) 386-394. DOI:10.1021/ic051483g |
| [9] |
A. Haber, I. Angel, A. Mahammed, Z. Gross, J. Diabetes Complications 27 (2013) 316-321. DOI:10.1016/j.jdiacomp.2013.02.005 |
| [10] |
L. Kupershmidt, Z. Okun, T. Amit, et al., J. Neurochem. 113 (2010) 363-373. DOI:10.1111/jnc.2010.113.issue-2 |
| [11] |
A. Mahammed, Z. Gross, Catal. Sci. Technol. 1 (2011) 535-540. DOI:10.1039/c1cy00063b |
| [12] |
R. Orłowski, D. Gryko, D.T. Gryko, Chem. Rev. 117 (2017) 3102-3137. DOI:10.1021/acs.chemrev.6b00434 |
| [13] |
H.Y. Liu, T.S. Lai, L.L. Yeung, C.K. Chang, Org. Lett. 5 (2003) 617-620. DOI:10.1021/ol027111i |
| [14] |
W. Shao, H. Wang, S. He, et al., J. Phys. Chem. B 116 (2012) 14228-14234. DOI:10.1021/jp306826p |
| [15] |
L. Shi, H.Y. Liu, H. Shen, et al., J. Porphyrins Phthalocyanines 13 (2009) 1221-1226. DOI:10.1142/S1088424609001546 |
| [16] |
L. You, H. Shen, L. Shi, et al., Sci. Chin. Phys. Mech. 53 (2010) 1491-1496. DOI:10.1007/s11433-010-4052-8 |
| [17] |
B.J. Brennan, Y.C. Lam, P.M. Kim, X. Zhang, G.W. Brudvig, ACS Appl. Mater. Interfaces 7 (2015) 16124-16130. DOI:10.1021/acsami.5b05050 |
| [18] |
J. Vestfrid, I. Goldberg, Z. Gross, Inorg. Chem. 53 (2014) 10536-10542. DOI:10.1021/ic501585a |
| [19] |
A. Mahammed, B. Tumanskii, Z. Gross, J. Porphyrins Phthalocyanines 15 (2011) 1275-1286. DOI:10.1142/S1088424611004191 |
| [20] |
J. Vestfrid, M. Botoshansky, J.H. Palmer, J. Am. Chem. Soc. 133 (2011) 12899-12901. DOI:10.1021/ja202692b |
| [21] |
G. Pomarico, L. Tortora, F.R. Fronczek, K.M. Smith, R. Paolesse, J. Inorg. Biochem. 158 (2016) 17-23. DOI:10.1016/j.jinorgbio.2016.02.005 |
| [22] |
T.H. Ngo, W. Van Rossom, W. Dehaen, W. Maes, Org. Biomol. Chem. 7 (2009) 439-443. DOI:10.1039/B819185A |
| [23] |
Q.Y. Chen, R.B. Du, C. Liu, D.M. Shen, Synlett 2009 (2009) 2701-2705. DOI:10.1055/s-0029-1217955 |
| [24] |
S. Nardis, F. Mandoj, R. Paolesse, et al., Eur. J. Inorg. Chem. 2007 (2007) 2345-2352. |
| [25] |
S. Nardis, G. Pomarico, F. Mandoj, et al., J. Porphyrins Phthalocyanines 14 (2010) 752-757. DOI:10.1142/S1088424610002513 |
| [26] |
B. Zyska, M. Schwalbe, Chem. Commun. 49 (2013) 3799-3801. DOI:10.1039/c3cc40625c |
| [27] |
R. Paolesse, Synlett 2008 (2008) 2215-2230. DOI:10.1055/s-2008-1078687 |
| [28] |
I. Saltsman, A. Mahammed, I. Goldberg, et al., J. Am. Chem. Soc. 124 (2002) 7411-7420. DOI:10.1021/ja025851g |
| [29] |
S. Nardis, G. Pomarico, M. Stefanelli, et al., J. Porphyrins Phthalocyanines 20 (2016) 465-474. DOI:10.1142/S1088424616500279 |
| [30] |
L. Tortora, S. Nardis, F.R. Fronczek, K.M. Smith, R. Paolesse, Chem. Commun. 47 (2011) 4243-4245. DOI:10.1039/c0cc05837h |
| [31] |
G.R. Geier, J.S. Lindsey, J. Porphyrins Phthalocyanines 06 (2002) 159-185. DOI:10.1142/S1088424602000208 |
| [32] |
M.H.R. Mahmood, H.Y. Liu, H.H. Wang, Y.Y. Jiang, C.K. Chang, Tetrahedron Lett. 54 (2013) 5853-5856. DOI:10.1016/j.tetlet.2013.08.086 |
| [33] |
J.Y. Shin, H. Furuta, K. Yoza, S. Igarashi, A. Osuka, J. Am. Chem. Soc. 123 (2001) 7190-7191. DOI:10.1021/ja0106624 |
| [34] |
B. Koszarna, D.T. Gryko, J. Org. Chem. 71 (2006) 3707-3717. DOI:10.1021/jo060007k |
| [35] |
Z.Y. Liu, M.H. Mahmood, J.Z. Wu, S.B. Yang, H.Y. Liu, Molecules 22 (2017). |
| [36] |
H.Y. Liu, F. Yam, Y.T. Xie, X.Y. Li, C.K. Chang, J. Am. Chem. Soc. 131 (2009) 12890-12891. DOI:10.1021/ja905153r |
| [37] |
H.Y. Liu, M.H.R. Mahmood, S.X. Qiu, C.K. Chang, Coord. Chem. Rev. 257 (2013) 1306-1333. DOI:10.1016/j.ccr.2012.12.017 |
| [38] |
C.K. Chang, C.Y. Yeh, T.S. Lai, Macromol. Symp. 156 (2000) 117-124. |
| [39] |
K.S. Suslick, M.M. Fox, J. Am. Chem. Soc. 105 (1983) 3507-3510. DOI:10.1021/ja00349a023 |
| [40] |
K.S. Suslick, B. Cook, M. Fox, J. Chem. Soc. Chem. Commun. (1985) 580-582. |
| [41] |
J.S. Lindsey, H.C. Hsu, I.C. Schreiman, Tetrahedron Lett. 27 (1986) 4969-4970. DOI:10.1016/S0040-4039(00)85109-6 |
| [42] |
J.S. Lindsey, I.C. Schreiman, H.C. Hsu, P.C. Kearney, A.M. Marguerettaz, J. Org. Chem. 52 (1987) 827-836. DOI:10.1021/jo00381a022 |
| [43] |
A.W. van der Made, E.J.H. Hoppenbrouwer, R.J.M. Nolte, W. Drenth, Recl. Trav. Chim. Pays-Bas 107 (1988) 15-16. |
| [44] |
Q. Zhang, F. Yang, Y. Wu, Org. Chem. Front. 1 (2014) 694-697. DOI:10.1039/C4QO00076E |
| [45] |
A. Scrivanti, V. Beghetto, U. Matteoli, et al., Tetrahedron Lett. 45 (2004) 5861-5864. DOI:10.1016/j.tetlet.2004.05.144 |
| [46] |
G. Pomarico, S. Nardis, M.L. Naitana, et al., Inorg. Chem. 52 (2013) 4061-4070. DOI:10.1021/ic400162y |
| [47] |
D. Gao, G. Canard, M. Giorgi, T.S. Balaban, Eur. J. Inorg. Chem. 2012 (2012) 5915-5920. DOI:10.1002/ejic.201201158 |
| [48] |
G. Pomarico, S. Nardis, R. Paolesse, et al., J. Org. Chem. 76 (2011) 3765-3773. DOI:10.1021/jo200026u |
| [49] |
G. Pomarico, S. Nardis, M. Stefanelli, et al., Inorg. Chem. 52 (2013) 8834-8844. DOI:10.1021/ic4010467 |
| [50] |
M. Stefanelli, M.L. Naitana, M. Chiarini, et al., Eur. J. Org. Chem. 2015 (2015) 6811-6816. DOI:10.1002/ejoc.v2015.31 |
| [51] |
A. Mahammed, M. Botoshansky, Z. Gross, Dalton Trans. 41 (2012) 10938-10940. DOI:10.1039/c2dt31261a |
| [52] |
S.M. Kuzmin, S.A. Chulovskaya, V.I. Parfenyuk, J. Porphyrins Phthalocyanines 19 (2015) 1053-1062. DOI:10.1142/S1088424615500807 |
| [53] |
D.M. Marin, S. Payerpaj, G.S. Collier, et al., Phys. Chem. Chem. Phys. 17 (2015) 29090-29096. DOI:10.1039/C5CP04359J |
| [54] |
J.H. Ha, G.Y. Jung, M.S. Kim, et al., Bull. Korean Chem. Soc. 22 (2001) 63-67. |
 2018, Vol. 29
2018, Vol. 29 

