b Department of Pharmacy, Qingdao Municipal Hospital, Qingdao 266001, China
Human thyroid stimulating hormone (TSH) is a 28, 000 Da glycoprotein hormone which is expressed by thyrotropin cells in the pituitary gland [1]. It helps maintaining normal functions of thyroid by stimulating the biosynthesis and secretion of thyroid hormones (triiodothyronine and thyroxine) in human serum [2]. In a health adult's blood, a reference concentration of TSH is 0.4-5.0 μIU/mL. Abnormal concentration of TSH in blood is a key index of thyroid disease. A concentration of TSH in serum below the low limit of the reference range may result in decreased basal metabolic rate, hypothermia and cold in tolerance [2, 3]. On the contrary, high TSH concentration in blood is the earliest laboratory manifestation of primary hypothyroidism, which can cause profound atrial fibrillation, mental retardation, decreased growth rate and reduced bonemineral density [4-6]. Therefore, it is imperative to develop rapid and reliable techniques to detect TSH for early intervention and prevention of thyroid disease.
Recently, many strategies for the detection of TSH have been reported, such as infrared spectroscopy [7], tandem mass spectrometry [8] and bioluminescent immunoassay [9]. Among them, electrochemical immunoassay is widely recognized as a promising strategy in terms of sensitivity, low cost and analysis speed. Electrochemical immunoassays measure the concentration of a substance using the reaction between an antigen and an antibody [10-12]. In order to improve the sensitivity of immunoassay sensors, signal amplification is usually applied [13, 14]. Recently, various advanced nanomaterials, such as carbon nanotubes (CNTs), graphene, and magnetic beads (MBs), have been used as the labels for signal amplification [14-17]. MBs are among the most widely used materials to improve the performance of immunosensing, because of the large specific surface areas for reaction and easy separation from reaction system [16, 18-20].
Miniaturization, integration and intelligentization are notable characteristics of modern analytical chemistry. Inkjet printed microchips with micro electro-mechanical system are valuable for the fabrication of various miniaturized analytical devices. Additionally, inkjet printed microchips could be used to increase the efficiency of immunological reactions due to the reduced assay time and small sample volume requirements [21-26]. For instance, a sensitive microchip-based electrochemical immunosensor was developed by our group for the determination of S100B, and the limit of detection could reach 0.1 pg/mL with the linear concentration range from 0.1 pg/mL to 100 pg/mL [27]. Marco's group used a novel impedimetric immunosensor based on an array of interdigitated-electrodes for atrazine detection, and obtained a limit of detection of 0.04 μg/L [28]. Ybarra's group developed an 8-channel-electrochemical cells-electrode platform for in-situ diagnosis of human and animal infectious diseases, especially Chagas disease [21].
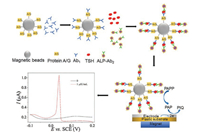
In this work, we propose a highly sensitive electrochemical method for TSH detection using an inkjet printed microchips with three-electrode and based on a double signal amplification strategy using protein A/G coated-magnetic beads (protein A/G@MBs), TSH antibodies (Ab1), alkaline phosphatase (ALP) labeled TSH antibodies (ALP-Ab2), and p-aminophenyl phosphate (pAPP). After the formation of a sandwich immunoaffinity structure between Ab1, TSH and ALP-Ab2, alkaline phosphatase (ALP) removes a phosphate group from pAPP to produce electroactive species p-aminophenol (pAP), which can be oxidized to p-quinone imine (QI) by electrode. Therefore, the detection of TSH is based on the amplification approaches using an enzymatic reaction and an electrochemical reaction. Compared with standard ELISA, the inkjet printed microchips offer a very promising approach due to the miniaturization, low sample consumption and rapid analysis. The immunoassay and the process of the experiment are shown as Fig. 1 and Fig. S1 in Supporting information, respectively. Protein A/G@MBs-Ab1 was prepared at first. Then 10 μL protein A/G@MBs-Ab1, 10 μL TSH at different concentrations and 10 μL diluted ALP-Ab2 by 10 mmol/L PBST 1/1000 were incubated for 45 min at 37 ℃ under 1000 rpm shaking to obtain the immunoassay protein A/G@MBs-Ab1-TSH-ALP-Ab2. The detail preparation was described in Supporting information.
|
Download:
|
| Fig. 1. Illustration of the protocol for the inkjet printed microchip immunosensor of TSH. | |
Alkaline phosphatase (ALP) is one of the most widely used reporter molecules for signal amplification in enzymatic reactions based detections because it can remove a phosphate group from substrates by hydrolyzing phosphoric acid monoesters into a phosphate ion and an electroactive molecule. The commonly used ALP substrate/product couples are L-ascorbic acid 2-phosphate (AAP)/L-ascorbic acid (AA), hydroquinone diphosphate (HQDP)/hydroquinone (HQ), 1-naphthyl phosphate (NPP)/1-naphthol (NP), and pAPP/pAP [29-31]. As illustrated in Fig. S2A in Supporting information, after the formation of a sandwich immunoaffiniy structure, the conjugated ALP can catalyze pAPP to pAP at pH 9.0. The typical electrochemical response for the immunosensor is also shown in Fig. S2A. An electrochemical response with two electrons and two protons is generated from the oxidation of pAP to p-quinonimide (pQI). As described in Fig. S2B, an irreversibly oxidized peak at 0.12 V formed by the electrochemical oxidation of pAP to pQI is only observed in curve a. Without pAPP, the peak is absent, as shown in curve b. It can be seen that an enzymatic reaction and an electrochemical reaction have occurred in this experiment leading to a signal amplification result.
To ensure an optimal analytical performance of the immunosensor, experimental parameters were studied. The effect of incubation time for protein A/G@MBs-Ab1 TSH and ALP-Ab2, and the concentration of ALP-Ab2 were evaluated by amperometric i-t curves carried out at 0.05 V with 5 μIU/mL TSH and 40 μL pAPP (60 μg/mL). It could be seen from Fig. 2A that the current increased with the increase of incubation time until reaching a maximum value at 45 min of incubation time and then decreased at 60 min of incubation time. Hence, an incubation time of 45 min was selected for the detection of TSH. Besides, various concentration of ALP-Ab2 of 1/500, 1/1000, 1/2000 and 1/4000 were tested. As shown in Fig. 2B, a decrease in current with the decrease of ALP-Ab2 concentration could be observed. Highest current was obtained at the concentrations of 1/500 and 1/1000. One/one thousand was selected as the optimal condition.

|
Download:
|
| Fig. 2. Effect of the incubation time for protein A/G@MBs-Ab1, TSH and ALP-Ab2 (A), different dilution ratios of ALP-Ab2 (B), the concentration of pAPP (C) and the reaction time for enzymatic reaction between pAPP and ALP (D) for the measurement of TSH (5 μIU/mL) by the immunosensor. | |
In addition, the concentration of pAPP and the reaction time for enzymatic reaction between pAPP and ALP were also optimized. DPV curves for 30 μg/mL, 60 μg/mL and 90 μg/mL pAPP were measured by the immunosensor with 5 μIU/mL TSH, 1/1000 ALP-Ab2, and a 45 min incubation time to form the sandwich immunocomplex. As illustrated in Fig. 2C, the oxidation currents increased with the increase of pAPP concentration from 30 μg/mL to 60 μg/mL, then decreased with the increase of pAPP concentration from 60 μg/mL to 120 μg/mL. The results indicated that the best concentration of pAPP was 60 μg/mL. Enzymatic reaction time was tested at 0, 10, 20 and 30 min when the immunosensor was incubated with 5 μIU/mL TSH, 1/1000 ALP-Ab2, a 45 min incubation time and was added into 40 μL pAPP (60 μg/mL). As shown in Fig. 2D, a highest current was observed when the the reaction time was 10 min.
To further confirm the ability of the biosensor for sensitive detection of TSH, DPV responses were measured with different concentrations of TSH under the optimal conditions. It could be observed on Fig. 3A that the DPV peak currents increased with the increase of TSH concentration from 0.01 μIU/mL to 10 μIU/mL. As presented in the inset of Fig. 3B, a good linear relationship between the DPV peak currents and the logarithm values of the target TSH concentrations ranging from 0.01 to 10 μIU/mL was obtained with a calibration function of I = 0.31logC + 0.99 (R2 = 0.99). The electrochemical measurements were repeated for each concentration of TSH for three times independently. The detection limit according to the 3 times of the standard deviation of the background was calculated to be 0.005 μIU/mL, which was lower than 0.02 μIU/mL based on the polyamidoamine-norfloxacin complex and core-shell Pd-Au hexoctahedrons electrochemiluminescence immunosensor [32] 0.014 μIU/mL obtained by the interdigitated microelectrodes biosensor [33] and 0.025 μIU/mL of a surface-enhanced Raman scattering-based lateral flow immunoassay sensor [34]. Such high sensitivity is due to (1) the ALP-based enzymatic-electrochemical reaction which leads to the remarkably amplified electrochemical signals and (2) the the signal carrier protein A/G MBs which can load a large amount of signal molecules to enhance the signal-to-noise ratio and to improve the detection sensitivity.

|
Download:
|
| Fig. 3. DPV responses (A) and calibration curves (B) for the electrochemical immunosensor corresponding to the analysis of different concentrations of target TSH. Inset (B): the linear relationship of currents versus the logarithm of target concentration in the range from 0.01 to 10 μIU/mL. Error bars showed the standard deviation of three experiments. | |
To evaluate the selectivity of the immunosensor, bovine serum albumin (BSA), thrombin or cytokeratin 19 (CK-19) at the concentrations of 1 nmol/L was mixed with 5 μIU/mL TSH for analysis. As shown in Fig. S3 in Supporting information, when the immunosensor was treated with the mixture (1 nmol/L BSA + 5 μIU/mL TSH, 1 nmol/L thrombin + 5 μIU/mL TSH or 1 nmol/L CK-19 + 5 μIU/mL TSH), the observed amperometric signals were almost the same as that obtained from only 5 μIU/mL TSH. The experimental results indicated that the proposed immunosensor performed an excellent selectivity for the detection of TSH. Additionally, the stability of the TSH immunosensor was investigated over a 20-day period by measuring 5 μIU/mL TSH. The sensor was stored at 4 ℃ when the immunosensor was not used. The tests were performed intermittently every 1-2 days in the beginning and then 4-5 days in the remaining days. The microchips retained 95%, 89% and 80% of their initial response after a storage period of 5, 10 and 20 days, respectively. The results indicated that the immunosensor had a good stability.
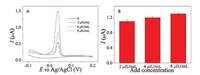
In order to test the applicability and reliability of the inkjet printed microchip immunosensor for clinical usage, the experiments were carried out in human serum samples containing different concentrations of TSH. The targets were analyzed in the same manner as described before. As shown in Fig. 4, the peak currents increase with the increase of TSH concentrations in human serum. According to Table S1 in Supporting information, the recoveries were from 98.0% to 101.8% and the relative standard deviations were from 1.3% to 3.1%, indicating that the immunosensor on an inkjet printed microchip provided a promising tool to detect TSH quantitatively in real clinical samples.
|
Download:
|
| Fig. 4. DPV responses of the immunosensor with the sample of human serum containing 2, 4 and 8 μIU/mL of TSH (A, B). | |
In this paper, we reported a sensitive electrochemical immunosensor coupling protein A/G@magnetic beads and an ALP-based enzymatic-electrochemical reaction on inkjet printed microchips for the determination of TSH. The use of protein A/G magnetic beads resulted in an increased sensitivity in TSH detection by enabling the immobilization of large amount of antibodies on the solid phase of the electrochemical transducer. Another signal amplification approach in this assay was the alkaline phosphatase and p-aminophenyl phosphate enzymatic-electrochemical reaction, which combined enzymatic reaction and electrochemical reaction. The immunosensor showed a detection limit of 0.005 μIU/mL. On the other hand, the present method is simple, specific and does not require sophisticated instrumentations. Moreover, the immunosensor showed potential applications in clinical analysis. It represents a compelling alternative to establish biological molecular detection techniques and opens a versatile way for discerning other hotspot mutations in clinical specimens.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (NSFC, Nos. 21775028, 21375022), and Science and Technology Commission of Shanghai Municipality (Nos.16391903900, 17JC1401900, 17JC1400200).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, inthe online version, at https://doi.org/10.1016/j.cclet.2018.01.042.
| [1] |
Y.L. Zhou, X.H. Xia, Y. Xu, et al., Anal. Chim. Acta 722 (2012) 95-99. DOI:10.1016/j.aca.2012.01.065 |
| [2] |
Y.T. Liu, Q.Q. Zhang, H.J. Wang, et al., Biosen. Bioelectron. 71 (2015) 164-170. DOI:10.1016/j.bios.2015.04.022 |
| [3] |
D.J. Zhou, T.S. Park, J.Y. Yoon, Biosen. Bioelectron. 40 (2013) 180-185. DOI:10.1016/j.bios.2012.07.014 |
| [4] |
X.Y. Wu, Y. Lu, H. Jiang, et al., Endocrine 46 (2014) 554-560. DOI:10.1007/s12020-013-0121-x |
| [5] |
R.N. Miguel, J. Sanders, Autoimmun. Highlights 8 (2017) 1-18. DOI:10.1007/s13317-016-0089-7 |
| [6] |
Z. Lin, X. Wang, Z.J. Li, et al., Talanta 75 (2008) 965-972. DOI:10.1016/j.talanta.2007.12.043 |
| [7] |
C. Mello, A. Marangono, R. Poppi, I. Noda, Anal. Chim. Acta 696 (2011) 47-52. DOI:10.1016/j.aca.2011.04.015 |
| [8] |
H.E. Deventer, D.R. Mendu, A.T. Remaley, S.J. Soldin, Clin. Chem. 57 (2011) 122-127. DOI:10.1373/clinchem.2010.154088 |
| [9] |
L.A. Frank, A.I. Petunin, E.S. Vysotski, Anal. Biochem. 325 (2004) 240-246. DOI:10.1016/j.ab.2003.11.003 |
| [10] |
B. Zhang, D.P. Tang, B.Q. Liu, et al., Anal. Chim. Acta 711 (2012) 17-23. DOI:10.1016/j.aca.2011.10.049 |
| [11] |
E.B. Bahadir, M.K. Sezginturk, Biosen. Bioelectron. 68 (2015) 62-71. DOI:10.1016/j.bios.2014.12.054 |
| [12] |
A.C. Glavan, D.C. Christodouleas, B. Mosadegh, et al., Anal. Chem. 86 (2014) 11999-12007. DOI:10.1021/ac5020782 |
| [13] |
B. Situ, N.N. Cao, B. Li, et al., Biosen. Bioelectron. 43 (2013) 257-263. DOI:10.1016/j.bios.2012.12.021 |
| [14] |
S.K.Y. Oaew, R. Charlemroj, T. Pattrakankul, N. Karoonuthaisiri, Biosen. Bioelectron. 34 (2012) 238-243. DOI:10.1016/j.bios.2012.02.011 |
| [15] |
E. Lahiff, C. Lynam, N. Gilmartin, R. O'Kennedy, D. Diamond, Anal. Bioanal. Chem. 398 (2010) 1575-1589. DOI:10.1007/s00216-010-4054-4 |
| [16] |
J. Richardson, P. Hawkins, R. Luxton, Biosen. Bioelectron. 16 (2001) 989-993. DOI:10.1016/S0956-5663(01)00201-9 |
| [17] |
H.J. Lin, Y.F. Liu, J.R. Huo, et al., Anal. Chem. 85 (2013) 6228-6232. DOI:10.1021/ac401075u |
| [18] |
V. Serafin, N. Ubeda, L. Agui, P. Yanez-Sedeno, J.M. Pingarron, Anal. Bioanal. Chem. 403 (2012) 939-946. DOI:10.1007/s00216-012-5753-9 |
| [19] |
X.Q. Zhong, L. Qiao, N. Gasilova, B.H. Liu, H.H. Girault, Anal. Chem. 88 (2016) 6184-6189. DOI:10.1021/acs.analchem.6b01142 |
| [20] |
G. Proczek, A.L. Gassner, J.M. Busnel, H.H. Girault, Anal. Bioanal. Chem. 402 (2012) 2645-2653. DOI:10.1007/s00216-011-5495-0 |
| [21] |
M.E. Cortina, L.J. Melli, M. Roberti, et al., Biosen. Bioelectron. 80 (2016) 24-33. DOI:10.1016/j.bios.2016.01.021 |
| [22] |
J.L. Li, K.W. Chang, C.H. Wang, et al., Biosen. Bioelectron. 79 (2016) 887-893. DOI:10.1016/j.bios.2016.01.029 |
| [23] |
K. Bravo, F. Ortega, G.A. Messina, et al., Clin. Chim. Acta 464 (2017) 64-71. DOI:10.1016/j.cca.2016.11.012 |
| [24] |
Y.Z. Wang, G.X. Zhu, W.J. Qi, Y. Li, Y.J. Song, Biosen. Bioelectron. 85 (2016) 777-784. DOI:10.1016/j.bios.2016.05.090 |
| [25] |
L.F. Cheow, S.H. Ko, S.J. Kim, K.H. Kang, J. Han, Anal. Chem. 82 (2010) 3383-3388. DOI:10.1021/ac9024335 |
| [26] |
K. Wilson, K. Homan, S. Emelianov, Nat. Commun. 3 (2012) 618-627. DOI:10.1038/ncomms1627 |
| [27] |
Y. Liu, H.X. Wang, J.Y. Chen, et al., Electroanalysis 25 (2013) 1050-1055. DOI:10.1002/elan.v25.4 |
| [28] |
J. Ramon-Azcon, E. Valera, A. Rodriguez, et al., Biosen. Bioelectron. 23 (2008) 1367-1373. DOI:10.1016/j.bios.2007.12.010 |
| [29] |
N. Xia, F.J. Ma, F. Zhao, et al., Electrochim. Acta 109 (2013) 348-354. DOI:10.1016/j.electacta.2013.07.118 |
| [30] |
H. Yang, Curr. Opin. Chem. Biol. 16 (2012) 422-428. DOI:10.1016/j.cbpa.2012.03.015 |
| [31] |
L. Liu, N. Xia, H.P. Liu, et al., Biosen. Bioelectron. 53 (2014) 399-405. DOI:10.1016/j.bios.2013.10.026 |
| [32] |
Y.T. Liu, Q.Q. Zhang, H.J. Wang, et al., Biosen. Bioelectron. 71 (2015) 164-170. DOI:10.1016/j.bios.2015.04.022 |
| [33] |
H.X. Wang, X.Z. Wu, P.T. Dong, et al., The 2013 Biomedical Engineering International Conference (2013).
|
| [34] |
S.J. Choi, J. Hwang, S. Lee, et al., Sens. Actuators B 240 (2017) 358-364. DOI:10.1016/j.snb.2016.08.178 |
 2018, Vol. 29
2018, Vol. 29 

