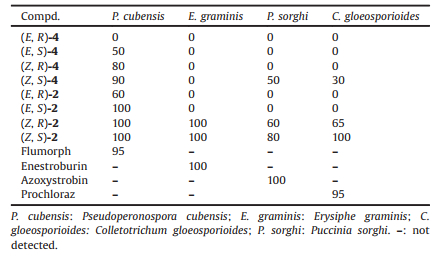
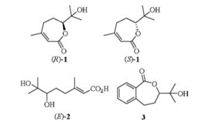
(6R)-3, 7-Dimethyl-7-hydroxy-2-octen-6-olide (R-1) (Fig. 1), which has an unique seven-membered lactone, and (2Z, 6R)-6, 7-dihydroxy-3, 7-dimethyloct-2-enoic acid (Z, R-2) (Fig. 2) were first isolated from the honey bee fungal entomopathogen Ascosphaera apis, as well as the fruit of plant Litsea cubeba in Tibet, and they exhibited good antifungal and antioxidant activities [1, 2]. This type of seven-membered lactone with α-hydroxy side chain was seldom found in nature, and the synthesis of which is unusual in literatures. 6, 7-Dihydroxy-3, 7-dimethyloct-2-enoic acid has been reported to be used in the treatment of skin lesion, however the olefin configuration, the absolute configuration of chiral center and its source were unknown [3]. 6, 7-Dihydroxy-3, 7-dimethyloct-2-enoic acid with the unknown olefin configuration, and absolute configuration of chiral center with very small optical rotation was also isolated from the root of Litsea cubeba and Amomum tsao-ko [4, 5]. In the previous paper, the racemic 3, 7-dimethyl-7-hydroxy-2-octen-6-olide (1), benzo analog 7-methyl-7-hydroxy-2, 3-benzo[c]octa-1, 6-olide (3) and (E)-6, 7-dihydroxy-3, 7-dimethyl-oct-2-enoic acid (E-2) (Fig. 1) were all synthesized via the epoxidation-lactonization approaches of olefin acid [6-8]. The (6R) and (6S) isomers (R-1 and S-1) were also synthesized with high ee values via Sharpless asymmetry dihydroxylation as the key steps due to its ability of construction chiral alcohol [9-15]. In order to compare the antifungal activity differences against phytopatho-gens and get insights into the relationship of the structures and antifungal activities between the seven-membered lactones, ring-opening olefin acids and olefin acid esters, the four stereoisomers of 6, 7-dihydroxy-3, 7-dimethyloct-2-enoic acid (Z, R-2, Z, S-2, E, R-2, E, S-2) and their methyl ester 4 (Fig. 2) were synthesized, and the in vivo antifungal activities were assayed in this paper.
|
Download:
|
| Fig. 1. The structures of seven-membered lactones and relating acid. | |

|
Download:
|
| Fig. 2. The isomers of 6, 7-dihydroxy-3, 7-dimethyloct-2-enoic acid and their esters. | |
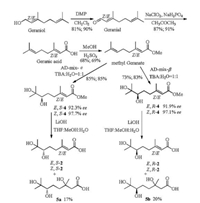
The synthetic strategy (Scheme 1) was initially investigated according to the procedures in the previous report [9]. First, cis-/trans-geraniols were oxidized to cis-/trans-geranials by DessMartin oxidant reagent (DMP). Then cis-/trans-geranials were further oxidized to cis-/trans-geranic acids with NaClO2 [6]. Because the acids could not react with the AD-mix-α/AD-mix-β directly, so the esters were prepared via the acids and methanol in the presence of concentrated H2SO4 at ambient temperature. Then the Sharpless asymmetry dihydroxylation of the esters were conducted with AD-mix-α and AD-mix-β to successfully give the chiral vicinal diol esters 4 (Figs. S1-8 and S65-70 in Supporting information) in 35.0%-48.0% overall yields with 91.9%-97.7% ee values, respectively. However, when they were hydrolyzed by base solution, only the mixtures of 2 and the Michael addition products 5 were afforded under various conditions such as LiOH, NaOH, KOH as the base, the water, water-methanol, water-THF, watermethanol-THF and isopropanol as the solvents. Unfortunately, even repeatedly performing the purification of the mixtures though preparative HPLC and TLC plates, we could not get a pure product 2 from the reaction mixture. Occasionally, the pure byproduct 5a and 5b were obtained in very low yield by preparative TLC plates and their structures were characterized by 1H NMR, 13C NMR (Figs. S9-12 in Supporting information) and high resolution mass spectrometry. Nonetheless, the absolute configuration of new chiral center was uncertain.
|
Download:
|
| Scheme 1. The synthetic route of 6, 7-dihydroxy-3, 7-dimethyloct-2-enoic acid by hydrolysis. | |
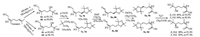
Because of the unsuccessful transformation from chiral esters to acids by hydrolysis, and construction chiral centers via Sharpless asymmetry dihydroxylation of cis- and trans-geranials due to their complexity of products in water media, then we have to change our synthetic strategy and go back to the raw materials (Schemes 2 and 3). To the best of our knowledge, trans-geraniol could be transferred to chiral triol mono acetate or N-phenylcarbamate, or geranyl chloride vic-diol by Sharpless asymmetry dihydroxylation, biological transformation or chemo-enzymatic hydrolysis with high ee values [16-21] (Scheme 2), whereas, these conversion approaches from these mono acetate or N-phenylcarbamate to chiral triol 6a and 6c need further exploration. On the other side, the resolution of chiral triol mono benzoate by formation and chromatographic separation of diastereoisomeric esters and further reduction with LiAlH4 to produce chiral triol 6a and 6c has also been reported [22]. All of these methods would take 3-5 steps and the overall yields were not satisfied. Although the selective direct dihydroxylation of geraniol double bond at allylic alcohol position by OsO4-TMEDA system at -78 ℃ was disclosed [23], the selective direct transformation at the remotedouble bond from geraniol to chiral triol 6a-6d was seldom found in references [24].
|
Download:
|
| Scheme 3. The synthetic route of 6, 7-dihydroxy-3, 7-dimethyloct-2-enoic acid isomers by Sharpless asymmetric dihydroxylation. | |
In this case, the direct transformation from geraniol to chiral triol was explored by repeated experiments in more details, and found that the Sharpless asymmetry dihydroxylation could be directly utilized to transfer both cis- and trans-geraniol to afford the key chiral triol intermediates 6a-6d in high yields (73%-83%) and high ee values (93.1%-96.1% ee) (Figs. S13-20 and S53-58 in Supporting information). The key chiral triol 6a-6d were easily protected by converting into the related acetonide derivatives 7a-7d using 2, 2-dimethoxypropane (2, 2-DMP) [25]. Then compounds 7a-7d were oxidized to aldehyde 8a-8d by DMP. After that, 8a-8d were further oxidized to cis-/trans-acids 9a-9d with NaClO2 [6]. All of these processes did not change the absolute configuration of the chiral centers. Finally, deprotection of 9a-9d by AcOH-H2O successfully gave the four isomers of the chiral hydroxyl olefin acids 2 in 32.6%-36.4% overall yields and 90.3%-97.5% ee values (Figs. S45-52, S59-64 and S73-76 in Supporting information) [26].
In the literatures, 6, 7-dihydroxy-3, 7-dimethyloct-2-enoic acid was isolated from the root of Litsea cubeba, and the configuration of its double bond was determined to E by the synthesis and comparison of its NMR data with the Z isomer [1, 4, 8]. However, the natural E isomer only had a [α]D20 - 0:8 (c 0.35, MeOH) optical rotation [4], we deduced that it was almost the racemic compound compared with the [α]D20 - 30:1 (c 1.2, MeOH) for the (E, S)-2, and [α]D20 + 26:3 (c 1.5, MeOH) for the (E, R)-2 synthesized in this paper.
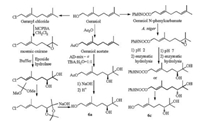
The in vivo fungicidal activity was evaluated according to the procedures (Section 1.2 in Supporting information) [27-29] and presented in Table 1. These data indicated that the chiral acids (Z, R)-2 and (Z, S)-2 and their esters have much higher activities with the inhibitory rates of 80%-100% than the chiral acid (E, R)-2 and itsester 4, the ester (E, S)-4 with the control rates of 0-60% against P. cubensis except (E, S)-2 with 100% inhibitory rate; the chiral acids (Z, R)-2 and (Z, S)-2 have much higher activities with the inhibitory rates of 60%-100% than the chiral acid (E, R)-2, (E, S)-2 and all four ester 4 with the inhibitory rates of 0-50% against E. graminis, P. sorghi and C. gloeosporioides. These data also showed that the chiral acids have better activities than their esters. The activities of the chiral acids with Z-configuration of double bond are better than those with E-configuration, and the activities of the chiral acids with S-configuration of chiral alcohol are better than those with R-configuration. Except against P. sorghi, the chiral acids (Z, S)-2 showed the same or even better activities against P. cubensis, E. graminis and C. gloeosporioides as that of the positive control. Based on the above data, the chiral acid (Z, S)-2 is a good lead compound with a broad spectrum of fungicidal activities, the configurations of the olefin's double bond and the chiral alcohol play a crucial rule for the fungicidal activities.
|
|
Table 1 The in vivo antifungal activity (inhibitory rate, %) of chiral acids 2 and esters 4 against several phytopathagens at 400 μg/mL. |
In conclusion, the four isomers of 6, 7-dihydroxy-3, 7-dimethyloct-2-enoic acid and their esters were synthesized via Sharpless asymmetry dihydroxylation as the key step in 35.0%-48.0% overall yields with 91.9%-97.7% ee values for esters 4 and 31.3%-36.4% overall yields with 90.3-97.5% ee values for acids 2, respectively, using cis- and trans-geraniol as the raw materials. Their structures were characterized by 1H NMR, 13C NMR and HR-ESI-MS data. The in vivo bioassay results showed that the chiral acids (Z, S)-2 is a good lead compound with 80%-100% inhibitory rates against P. cubensis, E. graminis, P. sorghi and C. gloeosporioides at 400 μg/mL.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21772229, 21172254) for the financial support. The bioassay section of the Sinochem Agrochemicals R & D Co., Ltd. at Shenyang performed the bioassay evaluation, and they were appreciated for their kind help and discussion.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.02.006.
| [1] |
L. Gabriela, N.I.P. Gallardo, G.M. Cabrera, Phytochem. Lett. 1 (2008) 155-158. DOI:10.1016/j.phytol.2008.07.008 |
| [2] |
Y. Yang, J.Z. Jiang, L.B. Qimei, et al., Molecules 15 (2010) 7075-7082. DOI:10.3390/molecules15107075 |
| [3] |
A. F. Burton, D. McLean, Patent US 5175190, 1992-12-29.
|
| [4] |
J. Chen, C.L. Zhu, H.Y. Xu, X. Ni, P.M. Yang, Chin. J. Pharm. 41 (2010) 504-508. |
| [5] |
K.Y. Lee, S.H. Kim, S.H. Sung, Planta Med. 74 (2008) 867-869. DOI:10.1055/s-2008-1074552 |
| [6] |
H.B. Dong, M.Y. Yang, J.Z. Jiang, M.A. Wang, J. Asian Nat. Prod. Res. 15 (2013) 880-884. DOI:10.1080/10286020.2013.803474 |
| [7] |
J. Zhao, H.B. Dong, M.Y. Yang, et al., J. Asian Nat. Prod. Res. 16 (2014) 312-317. DOI:10.1080/10286020.2013.879121 |
| [8] |
H.B. Dong, M.Y. Yang, B. Tang, M.A. Wang, Chin. J. Org. Chem. 34 (2014) 2350-2353. DOI:10.6023/cjoc201406005 |
| [9] |
M.A. Wang, H.B. Dong, M.Y. Yang, Patent CN 103012357A, 2012-12-28.
|
| [10] |
H.C. Kolb, M.S. Van Nieuwenhze, K.B. Sharpless, Chem. Rev. 94 (1994) 2483-2547. DOI:10.1021/cr00032a009 |
| [11] |
H.B. Dong, M.Y. Yang, X.T. Zhang, M.A. Wang, Tetrahedron:Asymmetry 25 (2014) 610-616. DOI:10.1016/j.tetasy.2014.03.006 |
| [12] |
M.M. Heravi, V. Zadsirjan, M. Esfandyari, T.B. Lashaki, Tetrahedron:Asymmetry 28 (2017) 987-1043. DOI:10.1016/j.tetasy.2017.07.004 |
| [13] |
X.Y. Jiang, X.H. Xu, F.L. Qing, Chin. Chem. Lett. 25 (2014) 111tent 5-1120. DOI:10.1016/j.cclet.2014.04.018 |
| [14] |
Q.F. Wang, F. Chen, Y. Zhao, X.Y. Li, S.Y. Zhang, Chin.Chem.Lett. 20 (2009) 763-766. DOI:10.1016/j.cclet.2009.02.018 |
| [15] |
D.G. Liu, J.J. Xue, Z.X. Xie, et al., Chin. Chem. Lett. 20 (2009) 775-778. DOI:10.1016/j.cclet.2009.03.026 |
| [16] |
X.M. Zhang, A. Archelas, R. Furstoss, J. Org. Chem. 56 (1991) 3814-3817. DOI:10.1021/jo00012a010 |
| [17] |
A.D. Fourneron, A. Archelas, R. Furstoss, J. Org. Chem. 54 (1989) 4686-4689. DOI:10.1021/jo00280a043 |
| [18] |
F. Felpin, C. Lory, H. Sow, S. Acherar, Tetrahedron 63 (2007) 3010-3016. DOI:10.1016/j.tet.2007.01.062 |
| [19] |
G. Vidari, A. Dapiaggi, G. Zanoni, L. Garlaschelli, Tetrahedron Lett. 34 (1993) 6485-6488. DOI:10.1016/0040-4039(93)85077-A |
| [20] |
J. Mori, M. Iwashima, M. Takeuchi, H. Saito, Chem. Pharm. Bull. 54 (2006) 391-396. DOI:10.1248/cpb.54.391 |
| [21] |
G. Edegger, S.F. Mayer, A. Steinreiber, K. Faber, Tetrahedron 60 (2004) 583-588. DOI:10.1016/j.tet.2003.10.106 |
| [22] |
R.B. Boar, K. Damps, J. Chem. Soc. Perkin Trans. I 8 (1977) 709-712. |
| [23] |
T.J. Donohoe, P.R. Moore, M.J. Waring, Tetrahedron Lett. 38 (1997) 5027-5030. DOI:10.1016/S0040-4039(97)01061-7 |
| [24] |
D. Xu, C.Y. Park, K.B. Sharpless, Tetrahedron Lett. 35 (1994) 2495-2498. DOI:10.1016/S0040-4039(00)77153-X |
| [25] |
C.Y. Chou, D.R. Hou, J. Org. Chem. 71 (2006) 9887-9890. DOI:10.1021/jo061767y |
| [26] |
J.S. Yadav, V. Vardhan, S. Das, Synthesis 46 (2014) 2347-2352. DOI:10.1055/s-00000084 |
| [27] |
A.Y. Guan, C.L. Liu, W. Chen, et al., J. Agric. Food Chem. 65 (2017) 1272-1280. DOI:10.1021/acs.jafc.6b05580 |
| [28] |
A.Y. Guan, M.A. Wang, W. Chen, et al., J. Fluorine Chem. 201 (2017) 49-54. DOI:10.1016/j.jfluchem.2017.08.008 |
| [29] |
A.Y. Guan, M.A. Wang, J.L. Yang, et al., J. Agric. Food Chem. 65 (2017) 10829-10835. DOI:10.1021/acs.jafc.7b03898 |
 2018, Vol. 29
2018, Vol. 29