b University of Chinese Academy of Sciences, Beijing 100049, China
Manganese(Ⅲ)-peroxo complexes have been suggested as key intermediates in the enzymatic cycles of Mn-containing enzymes, such as manganese superoxide dismutase (MnSOD) [1], catalase [2], and the oxygen-evolving complex (OEC) of photosystem Ⅱ [3]. In biomimetic studies, a variety of rationally designed heme and nonheme ligands have been applied in the synthesis of reactive manganese(Ⅲ)-peroxo complexes [4-9]. A notable example of the supporting ligands is the heme ligand TPP (TPP = tetraphenylporphyrin dianion), with which Valentine and co-workers obtained the first X-ray crystal structure of the manganese(Ⅲ)-peroxo complexes [10]. For nonheme systems, based on the facially-coordinating trispyrazolyl ligand TpiPr2 (TpiPr2 = hydridotris(3, 5-diisopropylpyrazolyl)borate anion), two more manganese(Ⅲ)-peroxo complexes were synthesized and structurally characterized by Moro-oka and co-workers [11, 12]. The nonheme macrocyclic TMC (TMC = N-tetramethylated cyclam) ligand series is versatile in the chemistry ofmetal-oxygen adducts, and several structurally characterized and highly reactivemanganese(Ⅲ)-peroxo complexes of TMC havebeen developed by Nam and co-workers [13-15].
The nonheme amino N-heteroarene ligands, such as aminopyridine, aminoquinoline and aminoimidazole ligand, have also been extensively investigated in the synthesis of manganese (Ⅲ)-peroxo complexes (see Fig. 1 for selected examples of aminopyridine ligand N4Py, aminoquinoline ligand L7q2, and aminoimidazole ligand imL52) [16-25]. On the other hand, the aminobenzimidazole ligand series, another member of the nonheme amino N-heteroarene ligand family, has been frequently used in the metal complex-catalyzed oxidation of organic substrates [26-35]. Recently, our group has succeeded in highly efficient (enantioselective) oxidation of hydrocarbons, olefins and alcohols with aqueous H2O2 as a terminal oxidant under mild conditions, in which the proline- derived aminobenzimidazole ligands play key roles in modulating the catalytic reactivity of the manganese complexes [26, 36-39].
|
Download:
|
| Fig. 1. Selected examples of nonheme amino N-heteroarene ligands for manganese (Ⅲ)-peroxo complexes. | |
On the basis of these above work, we are interested in the investigation of the activity of manganese(Ⅲ)-peroxo complex bearing an aminobenzimidazole supporting ligand. Herein, we report the synthesis and characterization of a novel manganese (Ⅲ)-peroxo complex supported by a proline-derived aminobenzimidazole ligand Pro3Bzim (Fig. 1). The corresponding manganese(Ⅲ)-peroxo complex is characterized by means of various spectroscopic methods, and its reactivity in aldehyde deformylation is also investigated.
We initiated our study by preparation of the precursor MnⅡ complex using an aminobenzimidazole ligand Pro3Bzim (Pro3Bzim = (S)-N, N', N'-tris(N-methylbenzimidazol-2-ylmethyl)-2-pyrrolidinemethanamine), which was readily synthesized from proline-derived diamine skeleton (S)-2-pyrrolidinemethanamine and 2-chloromethyl-N-methylbenzimidazole by following a modified procedure for the synthesis of its analogue Pro3Py (Pro3Py = (S)-N, N', N'-tris(2-picolinyl)-2-pyrrolidinemethanamine) in our previous report [40] (see the Supporting information for 1H and 13C NMR spectra of ligand Pro3Bzim). Reacting Pro3Bzim with equimolar amounts of Mn(OTf)2 in CH3CN at ambient temperature afforded the desired [MnⅡ(Pro3Bzim)(OTf)](OTf) complex (1-OTf) (Scheme 1).

|
Download:
|
| Scheme 1. Syntheses of precursor complex [MnⅡ(Pro3Bzim)(OTf)]+ (1) and manganese(Ⅲ)-peroxo complex [MnⅢ(Pro3Bzim)(O2)]+ (2). | |
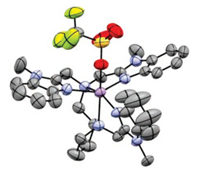
Single crystals of 1-OTf were obtained by diffusing ether into the CH3CN solution.X-raycrystallographic analysis reveals that the MnⅡ center resides in a distorted octahedral geometry and the Pro3Bzim ligand is coordinated to MnⅡ ion with five N donor atoms, two from the diamine backbone and three from the benzimidazole moieties. A triflate ligand is bound to the manganese center in the capacious apical position, indicating a labile site for the binding of peroxo ligand (See also Tables S1 and S2 in the Supporting information for crystallographic data of 1-OTf) (Fig. 2).
|
Download:
|
| Fig. 2. ORTEP plot for [MnⅡ(Pro3Bzim)(OTf)]+ (1; CCDC-1832630) with 50% probability thermal ellipsoids and H atoms omitted for clarity. Atom colors: Mn, violet; C, gray; N, blue; O, red; F, green; S, yellow. | |
With the MnⅡ starting material in hand, we set out to synthesize the corresponding MnⅢ-peroxo complex by following a conventional protocol [4]. Treating the colorless solution of 1 in CH3CN with H2O2 (40 equiv.) in the presence of triethylamine (5 equiv.) at 20 ℃ for about 2 min resulted in the completely formation of a pink species (2) with a characteristic ultraviolet–visible (UV-vis) absorption band at 540 nm (ε = ~140 L mol-1 cm-1, Fig. 3a), which is in agreement with the reported manganese(Ⅲ)-peroxo complexes [23]. It should be noted that 2 is metastable with a half-life about 0.5 h at 20 ℃ (see Fig. S3 in the Supporting information for the time profile of the natural decay of 2), and the metastable property has often been regarded as a characteristic feature of the reactive metal-oxygen adducts [41-44]. In order to identify the structure of 2, it was further characterized with coldspray ionization time-of-flight mass spectrometry (CSI-TOF MS) and continuous wave electron paramagnetic resonance (CW-EPR). The CSI-TOF-MS of 2 in positive mode exhibited a dominant ion peak at a mass-to-charge (m/z) ratio of 619.2, whose mass and isotope distribution pattern correspond to [MnⅢ(Pro3Bzim)(O2)]+ (calculated m/z 619.2) (Fig. 3b). When 2 was prepared with isotopically labeled H218O2 in the presence of triethylamine, a dominant ion peak corresponding to [MnⅢ(Pro3Bzim)(18O2)]+ appeared at m/z 623.2 (calculated m/z 623.2) (Fig. 3b, inset). The mass shift of 4 Daltons confirms that species 2 contains an O2 moiety in the coordination sphere. The X-band CW-EPR spectrum of a frozen solution of 2 appears silent, as expected for the MnⅢ oxidation state, which is in accordance with the manganese(Ⅲ)-peroxo complexes in literature [13, 14]. Based on the spectroscopic characterizations above, the new species 2 proves to be a manganese(Ⅲ)-peroxo complex, assigned as [MnⅢ(Pro3Bzim) (O2)]+. Given that the structure of ligand Pro3Bzim is similar to Pro3Py, taken together, it is likely that 2 possesses a similar coordination geometry to [MnⅢ(Pro3Py)(O2)]+ and the peroxo moiety is bound side-on as deduced for the latter and some other manganese(Ⅲ)-peroxo complexes [6, 40].

|
Download:
|
| Fig. 3. Spectroscopic characterizations of 2: (a) The changes of UV–vis absorption spectra showing the formation of 2 (red line) observed in the oxidation of 1 (1 mmol/L, blue line) by H2O2 (40 mmol/L) in the presence of triethylamine (5 mmol/L) in CH3CN at 20 ℃. Inset shows the time profile monitored at 540 nm for the formation of 2. (b) CSI-TOFMS spectrum of 2. Inset shows the isotope distribution patterns of 2-16O (blue line) and 2-18O (red line). (c) X-band CW-EPR spectrum of 2 recorded at 5 K. | |
Aldehyde deformylation is frequently used as a probe reaction to evaluate the reactivity of metal-peroxo complexes [4, 40, 45-50]. Therefore, we were inspired to examine the kinetic reactivity of 2 by using 2-phenylpropionaldehyde (2-PPA) as a probe substrate with UV–vis spectrophotometry. Upon addition of 2-PPA to 2 in CH3CN at 20 ℃, the absorption band at 540 nm, the typical absorption of manganese(Ⅲ)-peroxo 2, decreased with a pseudofirst-order kinetics profile (Fig. 4a). GC-MS analysis of the resulting solution revealed the formation of acetophenone as the sole product, as observed in the deformylation of 2-PPA by other metalperoxo complexes [15, 40, 45-49]. The first-order rate constant (kobs), determined by pseudo-first-order fitting of the kinetic data for the decay of 2 monitored at 540 nm, increased linearly with increasing concentration of 2-PPA, thereby giving a second-order rate constant (k2) of 0.10 L mol-1 s-1 at 20 ℃ (Fig. 4b). This reaction rate is comparable with the reported manganese(Ⅲ) -peroxo complexes [15, 40, 50]. Thus, we demonstrate that 2 is capable of performing oxidative deformylation reaction of aldehyde with second-order kinetics.

|
Download:
|
| Fig. 4. Kinetics of the reaction of 2 with 2-PPA: (a) UV–vis absorption spectral changes observed in the reaction of 2 (1 mmol/L, red line) with 2-PPA (90 mmol/L) in CH3CN at 20 ℃. Inset shows the time profile of 2 monitored at 540 nm obeying pseudo-first-order kinetics. (b) Plot of the first-order rate constant (kobs) against the concentration of 2-PPA to determine the second-order rate constant (k2) in the reaction of 2-PPA with 2 (1 mmol/L) in CH3CN at 20 ℃. | |
In summary, we have employed a proline-derived pentadentate aminobenzimidazole ligand (Pro3Bzim) to synthesize the N5-MnⅡ complex (1), which was crystallographically characterized and further used in the preparation of a novel manganese(Ⅲ)-peroxo complex (2) by a conventional protocol with H2O2 in the presence of triethylamine. The structure of 2 was well characterized with various spectroscopic techniques, including UV-vis, CSI-TOF MS and CW-EPR, while the reactivity of 2 was investigated in oxidative deformylation of aldehyde, demonstrating second-order kinetics in the reaction with 2-phenylpropionaldehyde and affording acetophenone as the sole product. The aminobenzimidazole ligands have proven to be excellent candidates in the development of enzyme models toward to catalytic oxidation reactions. The present work provides valuable example for this type ligands in the study of synthetic metal-oxygen adducts. Further investigations focusing on the development of more effective and versatile amino N-heteroarene ligands are underway in our lab.
AcknowledgmentsThis work is financially supported by the National Natural Science Foundation of China (No. 21473226) and Natural Science Foundation of Jiangsu Province (No. BK20161261). We acknowledge Prof. Wonwoo Nam (Ewha Womans University, Korea) for the measurements of CSI-TOF MS and CW-EPR.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.04.036.
| [1] |
Y. Sheng, I.A. Abreu, D.E. Cabelli, et al., Chem. Rev. 114 (2014) 3854-3918. DOI:10.1021/cr4005296 |
| [2] |
A.J. Wu, J.E. Penner-Hahn, V.L. Pecoraro, Chem. Rev. 104 (2004) 903-938. DOI:10.1021/cr020627v |
| [3] |
J.P. McEvoy, G.W. Brudvig, Chem. Rev. 106 (2006) 4455-4483. DOI:10.1021/cr0204294 |
| [4] |
J. Cho, R. Sarangi, W. Nam, Acc. Chem. Res. 45 (2012) 1321-1330. DOI:10.1021/ar3000019 |
| [5] |
K.P. Bryliakov, E.P. Talsi, Coord. Chem. Rev. 276 (2014) 73-96. DOI:10.1016/j.ccr.2014.06.009 |
| [6] |
D.F. Leto, T.A. Jackson, J. Biol. Inorg. Chem. 19 (2014) 1-15. |
| [7] |
J.A. Kovacs, Acc. Chem. Res. 48 (2015) 2744-2753. DOI:10.1021/acs.accounts.5b00260 |
| [8] |
X.F. Li, J.Y. Liu, Z.X. Guo, et al., Chin. Chem. Lett. 12 (2001) 979-982. |
| [9] |
L. Yu, Q. Wang, L. Dai, et al., Chin. Chem. Lett. 24 (2013) 447-449. DOI:10.1016/j.cclet.2013.03.029 |
| [10] |
R.B. VanAtta, C.E. Strouse, L.K. Hanson, et al., J. Am. Chem. Soc. 109 (1987) 1425-1434. DOI:10.1021/ja00239a024 |
| [11] |
N. Kitajima, H. Komatsuzaki, S. Hikichi, et al., J. Am. Chem. Soc. 116 (1994) 11596-11597. DOI:10.1021/ja00104a061 |
| [12] |
U.P. Singh, A.K. Sharma, S. Hikichi, et al., Inorg. Chim. Acta 359 (2006) 4407-4411. DOI:10.1016/j.ica.2006.06.033 |
| [13] |
M.S. Seo, J.Y. Kim, J. Annaraj, et al., Angew. Chem. Int. Ed. 46 (2007) 377-380. DOI:10.1002/(ISSN)1521-3773 |
| [14] |
J. Annaraj, J. Cho, Y.M. Lee, et al., Angew. Chem. Int. Ed. 48 (2009) 4150-4153. DOI:10.1002/anie.v48:23 |
| [15] |
H. Kang, J. Cho, K.B. Cho, et al., Chem.-Eur. J. 19 (2013) 14119-14125. DOI:10.1002/chem.201301641 |
| [16] |
R.L. Shook, W.A. Gunderson, J. Greaves, et al., J. Am. Chem. Soc. 130 (2008) 8888-8889. DOI:10.1021/ja802775e |
| [17] |
R.L. Shook, A.S. Borovik, Inorg. Chem. 49 (2010) 3646-3660. DOI:10.1021/ic901550k |
| [18] |
R.A. Geiger, S. Chattopadhyay, V.W. Day, et al., J. Am. Chem. Soc. 132 (2010) 2821-2831. DOI:10.1021/ja910235g |
| [19] |
R.A. Geiger, S. Chattopadhyay, V.W. Day, et al., Dalton Trans. 40 (2011) 1707-1715. DOI:10.1039/c0dt01570a |
| [20] |
R.A. Geiger, D.F. Leto, S. Chattopadhyay, et al., Inorg. Chem. 50 (2011) 10190-10203. DOI:10.1021/ic201168j |
| [21] |
R.A. Geiger, G.B. Wijeratne, V.W. Day, et al., Eur. J. Inorg. Chem (2012) 1598-1608. |
| [22] |
D.F. Leto, S. Chattopadhyay, V.W. Day, et al., Dalton Trans. 42 (2013) 13014-13025. DOI:10.1039/c3dt51277k |
| [23] |
S. El Ghachtouli, H.Y. Ching, B. Lassalle-Kaiser, et al., Chem. Commun. 49 (2013) 5696-5698. DOI:10.1039/c3cc41300d |
| [24] |
S. Groni, G. Blain, R. Guillot, et al., Inorg. Chem. 46 (2007) 1951-1953. DOI:10.1021/ic062063+ |
| [25] |
S. Groni, P. Dorlet, G. Blain, et al., Inorg. Chem. 47 (2008) 3166-3172. DOI:10.1021/ic702238z |
| [26] |
B. Wang, S. Wang, C. Xia, et al., Chem.-Eur. J. 18 (2012) 7332-7335. DOI:10.1002/chem.201200992 |
| [27] |
X. Wang, C. Miao, S. Wang, et al., ChemCatChem 5 (2013) 2489-2494. DOI:10.1002/cctc.201300102 |
| [28] |
C. Miao, C. Xia, W. Sun, Sci. Sin. Chim. 44 (2014) 1865-1875. DOI:10.1360/N032014-00199 |
| [29] |
D. Shen, C. Miao, S. Wang, et al., Eur. J. Inorg. Chem (2014) 5777-5782. |
| [30] |
X. Wang, C. Miao, S. Wang, et al., J. Mol. Catal. 28 (2014) 204-209. |
| [31] |
M. Mitra, J. Lloret-Fillol, M. Haukka, et al., Chem. Commun. 50 (2014) 1408-1410. DOI:10.1039/C3CC47830K |
| [32] |
O. Cussó, M. Cianfanelli, X. Ribas, et al., J. Am. Chem. Soc. 138 (2016) 2732-2738. DOI:10.1021/jacs.5b12681 |
| [33] |
M. Milan, M. Bietti, M. Costas, ACS Cent. Sci. 3 (2017) 196-204. DOI:10.1021/acscentsci.6b00368 |
| [34] |
M. Milan, G. Carboni, M. Salamone, et al., ACS Catal. 7 (2017) 5903-5911. DOI:10.1021/acscatal.7b02151 |
| [35] |
X.L. Liu, Q.Y. Cheng, X. Yang, et al., Chin. Chem. Lett. 12 (2001) 563-564. |
| [36] |
B. Wang, C. Miao, S. Wang, et al., Chem.-Eur. J. 18 (2012) 6750-6753. DOI:10.1002/chem.201103802 |
| [37] |
D. Shen, C. Miao, S. Wang, et al., Org. Lett. 16 (2014) 1108-1111. DOI:10.1021/ol4037083 |
| [38] |
D. Shen, C. Miao, D. Xu, et al., Org. Lett. 17 (2015) 54-57. DOI:10.1021/ol5032156 |
| [39] |
B. Qiu, D. Xu, Q. Sun, et al., ACS Catal. 8 (2018) 2479-2487. DOI:10.1021/acscatal.7b03601 |
| [40] |
J. Du, D. Xu, C. Zhang, et al., Dalton Trans. 45 (2016) 10131-10135. DOI:10.1039/C6DT00508J |
| [41] |
K. Ray, F.F. Pfaff, B. Wang, et al., J. Am. Chem. Soc. 136 (2014) 13942-13958. DOI:10.1021/ja507807v |
| [42] |
W.N. Oloo, L. Que Jr, Acc. Chem. Res. 48 (2015) 2612-2621. DOI:10.1021/acs.accounts.5b00053 |
| [43] |
J. Serrano-Plana, I. Garcia-Bosch, A. Company, et al., Acc. Chem. Res. 48 (2015) 2397-2406. DOI:10.1021/acs.accounts.5b00187 |
| [44] |
R.A. Baglia, J.P.T. Zaragoza, D.P. Goldberg, Chem. Rev. 117 (2017) 13320-13352. DOI:10.1021/acs.chemrev.7b00180 |
| [45] |
J. Annaraj, Y. Suh, M.S. Seo, et al., Chem. Commun (2005) 4529-4531. |
| [46] |
J. Cho, S. Jeon, S.A. Wilson, et al., Nature 478 (2011) 502-505. DOI:10.1038/nature10535 |
| [47] |
J. Cho, R. Sarangi, H.Y. Kang, et al., J. Am. Chem. Soc. 132 (2010) 16977-16986. DOI:10.1021/ja107177m |
| [48] |
J. Kim, B. Shin, H. Kim, et al., Inorg. Chem. 54 (2015) 6176-6183. DOI:10.1021/acs.inorgchem.5b00294 |
| [49] |
F.G. Cantu' Reinhard, P. Barman, G. Mukherjee, et al., J. Am. Chem. Soc. 139 (2017) 18328-18338. DOI:10.1021/jacs.7b10033 |
| [50] |
C.M. Lee, C.H. Chuo, C.H. Chen, et al., Angew. Chem. Int. Ed. 51 (2012) 5427-5430. DOI:10.1002/anie.201201735 |
 2018, Vol. 29
2018, Vol. 29 

