b First Affiliated Hospital of Southern University of Science and Technology, Shenzhen People's Hospital & Shenzhen Interventional Medical Engineering Research and Development Center, Shenzhen 518020, China;
c Beijing University of Chemical Technology, Beijing 100029, China;
d Beijing Technology & Business University, Beijing Higher Institution Engineering Research Center of Food Additives and Ingredients, Beijing Key Laboratory of Flavor Chemistry, Beijing Laboratory for Food Quality and Safety, Beijing 100048, China;
e University of Chinese Academy of Sciences, Beijing 100049, China
Superoxide dismutase (SOD) is an active substance that eliminates harmful substances produced by organisms during their metabolism. SOD is a natural scavenger of oxygen free radicals in organisms and has a wide range of medical value [1, 2]. The extraction of SOD is mostly from animal blood, which is not only expensive, but also exclusive of animal SOD, difficult to store at room temperature, cross-infection of blood viruses such as Acquired Immune Deficiency Syndrome (AIDS), and other potential risks. The International Health Organization has called for the immediate cessation of the use of animal SOD. The research on extracting SOD from plants has arisen. However, due to the high cost and difficulty to produce it, SOD cannot play its role widely and universally [3]. It is a trend to replace extraction of SOD from organism with other safer and more economical synthesis methods.
In recent years, the rise of nanomaterials has attracted widespread attention and promoted the process of nanomaterial-based artificial enzymes, which are defined as "nanozymes" [4, 5]. The scientists found that the inorganic nanomaterial Fe3O4 has intrinsic catalytic activity similar to horseradish peroxidase (HRP), which is an early period of studying nanozymes [6]. Nanozymes are low cost, easy for mass-production, highly stable and tunable. Researchers have discovered that they are better than natural enzymes in some conditions and that they can be used more widely in many aspects, from biosensing and immunoassays, to stem cell growth and pollutant removal [7-9]. More and more nanoparticles (magnetic materials, noble metal nanoclusters, metal oxide nanoparticles, and carbon based nanostructures) have been found to exhibit intrinsic enzyme activities, and the most of the nanozymes' catalytic reactions are based on HRP [10-12]. Some progress has also been made in the study of SOD nanozymes. Cerium oxide-based nanomaterials [13], metal-based nanomaterials [14] and carbon-based nanomaterials [15, 16] have been discovered about their SOD mimic abilities. However, since the artificial enzymes own the common gender that they are limited in catalyzing reactions, low selectivity and inadequate uses in realistic nanomedicine and biosystem, a great amount of new powerful nanozymes still need to be discovered.
Mn-doped ZnS quantum dots (QDs) is an important member of Ⅱ–Ⅵ compound semiconductors which have been extensively investigated for various applications other than biomedical labeling, such as displays, sensors, and lasers due to their low material cost, simple synthesis process, good water solubility, excellent chemical stability, and especially lower toxicity than QDs [17-19]. To the best of our knowledge, these studies usually focus on the fluorescence properties of Mn-doped ZnS QDs [20, 21]. The nanozymes property of them has not been reported so far.
In this work, we found that Mn-doped ZnS QDs possess intrinsic SOD-like activity. The experiments show that the reaction rate of autoxidation of pyrogallol was reduced with Mn-doped ZnS QDs increased. The Mn-doped ZnS QDs could catalyze the dismutation reaction of O2·-, similar to SOD. The influences of temperature and pH on the activity of Mn-doped ZnS QDs and SOD enzymes have been investigated. The ZnS QDs with the same and different synthetic methods all exhibited lower SOD-like activities than Mndoped ZnS QDs, which indicates that this is the inherent property of ZnS QDs. Compared with the extraction of SOD and other mimetic enzymes, Mn-doped ZnS QDs nanozymes have the advantages of high yield, simple synthesis process and good biocompatibility, which has a good application prospect.
Mn-doped ZnS QDs was synthesized in aqueous solution based on a previous publication with minor modifications [22]. First, 12.5 mmol of ZnSO4·7H2O and 1 mmol of MnCl2·4H2O was dissolved in 40 mL of deionized water. The mixture was stirred constantly for 20 min in a nitrogen atmosphere. After that, a Na2S·9H2O solution (20 mL, 0.6 mol/L) was dropped into the mixture while stirring for another 1 h. The product were centrifuged and washed thoroughly with deionized water and absolute ethanol, and finally dispersed in 50 mL deionized water.
The typical TEM image of Mn-doped ZnS QDs was shown in Fig. 1A. The Mn-doped ZnS QDs were nearly spherical and there was a little aggregation of particles. The size distribution of Mndoped ZnS QDs was shown in Fig. 1B. The maximum population was estimated to be 28 nm. The Mn-doped ZnS QDs emitted obvious orange-red fluorescence under UV light (365 nm) while appearing as white emulsus solution with good dispersibility under daylight (inset of Fig. 1C). The prepared Mn-doped ZnS QDs had an emission at 600 nm when excited at 350 nm (Fig. 1C).

|
Download:
|
| Fig. 1. (A) TEM images of Mn-doped ZnS QDs. (B) The diameter histogram of the Mn-doped ZnS QDs. (C) Emission spectra of Mn-doped ZnS QDs, excited at 350 nm. Inset: photographs of Mn-doped ZnS QDs under daylight lamp and UV light by a 365 UV lamp. (D) The viability of Hep G2 cells with different concentrations of Mn-doped ZnS QDs. | |
The biocompatibility of Mn-doped ZnS QDs was assessed by the cytotoxicity of Mn-doped ZnS QDs investigated. The methylthiazol tetrazolium (MTT) assay with Hep G2 cells result indicated that the viability of cells remained unchanged when the cells were exposed to Mn-doped ZnS QDs in the range of 0–500 μg/mL (Fig. 1D). The cell viability was still nearly 90% even after incubation for 24 h at the highest tested concentration of 500 μg/mL. This result suggests that Mn-doped ZnS QDs are highly biocompatible.
To our surprise, further studies revealed that Mn-doped ZnS QDs exhibit SOD-like catalytic activity. The SOD activity of Mndoped ZnS QDs has been investigated by inhibiting the autoxidation of pyrogallol (Improved Marklund method). The pyrogallol can rapidly self-oxidize under alkaline conditions and release O2·- to produce colored intermediates. After the start of the reaction, the pyrogallol solution changed to yellow brown for several minutes and then turned green for several hours and then turned into yellow. This is due to the fact that the resulting intermediate is continuously oxidized. When there was a presence of SOD, it can prevent the accumulation of intermediates because it can catalyze the formation of O2 and H2O2 by combining O2·- and H+. The enzyme activity of SOD was determined by observing the absorbance at 325 nm. Therefore, the low absorption values of the intermediate product means the high activity of SOD. Fig. 2A showed the absorption of the pyrogallol buffer solution (pH 8.2) with or without Mn-doped ZnS QDs. As can be seen, there was good linear relationship between the difference of absorption values of the autoxidation of pyrogallol system and reaction time. The values were obviously decreased after adding the Mn-doped ZnS QDs. Furthermore, the absorption values decreased successively with increasing Mn-doped ZnS QDs concentration. This proved that the reaction rate of autoxidation of pyrogallol was reduced with Mndoped ZnS QDs. All these observations indicated that Mn-doped ZnS QDs exhibit SOD-like catalytic activity.

|
Download:
|
| Fig. 2. (A) The time-dependent absorbance changes at 325 nm of SOD activity detection system by improved Marklund method with the absence or presence of various concentrations of Mn-doped ZnS QDs. (B) The time-dependent absorbance changes at 550 nm of SOD activity detection system by McCord method with the absence or presence of various concentrations of Mn-doped ZnS QDs. | |
We also evaluated the SOD-like activity of Mn-doped ZnS QDs by McCord method. Under the aerobic condition, xanthine oxidase catalyzes the oxidation of xanthine to produce uric acid and produces O2·-. At the same time, oxidized cytochrome c is reduced to reduced cytochrome c. The latter has a maximum absorption at 550 nm. In the presence of SOD, O2·- catalyzed and disproportionated cytochrome c reduction rates were reduced. In view of the similarity principle, we determined the activity of Mn-doped ZnS QDs according to the rate of change of the cytochrome c by O2·- reduction before and after the addition of various concentrations of Mn-doped ZnS QDs (Fig. 2B). After adding Mn-doped ZnS QDs to 0.2 mg/mL, the absorption intensity decreased significantly compared with that without adding Mn-doped ZnS QDs. It also displays that Mn-doped ZnS QDs showed the high SOD-like activity.
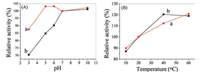
The performance of Mn-doped ZnS QDs was compared with natural SOD. As shown in Fig. 3, the SOD-like activity of Mn-doped ZnS QDs has been measured while varying the pH from 3 to 10 and the temperature from 10 ℃ to 60 ℃, and compared the results with the activity found in SOD over the same range of parameters. Alkaline conditions have little effect on the activity of both Mndoped ZnS QDs and natural SOD (Fig. 3A). However, the acid environment has a great influence on the activity of natural SOD. The relative activity of SOD decreased continuously as pH values decreased. By comparison, Mn-doped ZnS QDs shows better acid resistance. The activities of Mn-doped ZnS QDs remained intact even at pH 5. So, Mn-doped ZnS QDs can be used in a wider range of pH values (5 to 10). The Mn-doped ZnS QDs could make up the deficiency of SOD under the acidic conditions, and this could be expected to bring new application fields. Fig. 3B shows that the activities of Mn-doped ZnS QDs increased as the temperature became higher, which were very similar to the values for SOD. The activities change of Mn-doped ZnS QDs was larger than natural SOD in high temperature, indicating that the temperature was less affected. Furthermore, the SOD-like activity of Mn-doped ZnS QDs after storage at room temperature for six months was only little changed (Fig. S1 in Supporting information).
|
Download:
|
| Fig. 3. Dependency of the Mn-doped ZnS QDs (a) or SOD (b) on pH (A) and temperature (B). The usual conditions (pH 7, 20 ℃) point in each curve was set as 100%. | |
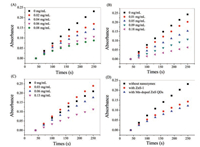
SOD is an active protease containing metal elements. Zinc, manganese, copper, and iron are all metal cofactors of SOD. The activity of SOD enzyme is closely related to these metal prosthetic groups [23, 24]. We speculate that the nature of Mn-doped ZnS QDs mimic enzyme is related to its composition. The SOD activity of ZnS QDs without manganese synthesized by the same method has been investigated (named ZnS-1 and its synthesis method is shown in Supporting information). As shown in Fig. 4A, the absorption intensity of mixture at 325 nm decreases gradually with the increase of ZnS-1 concentration, which indicating that the oxidation rate of pyrogallol slows down. The ZnS-1 also exhibit SOD-like catalytic activity. In order to investigate the influence of synthetic methods, the mimetic enzyme properties of ZnS synthesized by different methods were studied (named ZnS-2 and ZnS-3. Their synthesis methods are shown in Fig. S2 in Supporting information). The autoxidation of pyrogallol experiments showed that all of these ZnS nanoparticles could reduce the reaction rate of autoxidation of pyrogallol (Figs. 4B and C). The synthetic method did not affect the mimetic enzymes properties of ZnS nanoparticles. Furthermore, Mn-doped ZnS QDs inhibited the autoxidation of pyrogallol more significantly compared with ZnS-1, when using the same amount of ZnS-1 and Mn-doped ZnS QDs (Fig. 4D). This is probably because manganese is also an active element of SOD.
|
Download:
|
| Fig. 4. (A) The time-dependent absorbance changes at 325 nm of SOD activity detection system with the absence or presence of various concentrations of ZnS-1. (B) The timedependent absorbance changes at 325 nm of SOD activity detection system with the absence or presence of various concentrations of ZnS-2. (C) The time-dependent absorbance changes at 325 nm of SOD activity detection system with the absence or presence of various concentrations of ZnS-3. (D) The time-dependent absorbance changes at 325 nm of SOD activity detection system with the absence or presence of 0.04 mg/mL ZnS-1 or Mn-doped ZnS QDs. | |
In conclusion, the Mn-doped ZnS QDs with SOD-like activities were synthesized by a facile and one-pot method. The activity of Mn-doped ZnS QDs as SOD mimics is evaluated using the improved Marklund and McCord method. The Mn-doped ZnS QDs show more resistant to pH than SOD, especially in acidic environments. The Mn-doped ZnS QDs can still keep good mimic enzyme activity at room temperature for 6 months. The results showed that ZnS had the activity of SOD like enzyme, but weaker than Mn-doped ZnS QDs. This Mn-doped ZnS QDs nanozymes with low cost, simple synthesis, white, non-toxic provides a new promising application material for the application of SOD.
AcknowledgmentWe acknowledge the National Natural Science Foundation of China (Nos. 61571426, 61671435, 81630053), and Beijing Natural Science Foundation (No. 4161003) for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.12.007.
| [1] |
M. Balamurugan, P. Santharaman, T. Madasamy, et al., Biosens. Bioelectron. 116 (2018) 89-99. DOI:10.1016/j.bios.2018.05.040 |
| [2] |
Y. Wang, R. Branicky, A. Noe, S. Hekimi, J. Cell Biol. 217 (2018) 1915-1928. DOI:10.1083/jcb.201708007 |
| [3] |
F. Dashtestani, H. Ghourchian, K. Eskandari, H.A. Rafiee-Pour, Microchim. Acta 182 (2015) 1045-1053. DOI:10.1007/s00604-014-1424-1 |
| [4] |
H. Wei, E. Wang, Chem. Soc. Rev. 42 (2013) 6060-6093. DOI:10.1039/c3cs35486e |
| [5] |
Z.W. Chen, J.J. Yin, Y.T. Zhou, et al., ACS Nano 6 (2012) 4001-4012. DOI:10.1021/nn300291r |
| [6] |
L. Gao, J. Zhuang, L. Nie, et al., Nat. Nanotechnol. 2 (2007) 577-583. DOI:10.1038/nnano.2007.260 |
| [7] |
K. Fan, C. Cao, Y. Pan, et al., Nat. Nanotechnol. 7 (2012) 459-464. DOI:10.1038/nnano.2012.90 |
| [8] |
X. Ren, J. Liu, J. Ren, F. Tang, X. Meng, Nanoscale 7 (2015) 19641-19646. DOI:10.1039/C5NR04685H |
| [9] |
L. Feng, Z. Dong, C. Liang, et al., Biomaterials 181 (2018) 81-91. DOI:10.1016/j.biomaterials.2018.07.049 |
| [10] |
H. Jiang, Z. Chen, H. Cao, Y. Huang, Analyst 137 (2012) 5560-5564. DOI:10.1039/c2an35911a |
| [11] |
M. Shamsipur, A. Safavi, Z. Mohammadpour, Sensor. Actuat. B-Chem. 199 (2014) 463-469. DOI:10.1016/j.snb.2014.04.006 |
| [12] |
H. Sun, A. Zhao, N. Gao, et al., Angew. Chem. 54 (2015) 7176-7180. DOI:10.1002/anie.201500626 |
| [13] |
Y. Li, X. He, J.J. Yin, et al., Angew. Chem. 54 (2015) 1832-1835. DOI:10.1002/anie.201410398 |
| [14] |
M. Moglianetti, E.D. Luca, D. Pedone, et al., Nanoscale 8 (2016) 3739-3752. DOI:10.1039/C5NR08358C |
| [15] |
Z.Q. Xu, J.Y. Lan, J.C. Jin, et al., ACS Appl. Mater. Inter. 7 (2015) 28346-28352. DOI:10.1021/acsami.5b08945 |
| [16] |
X. Ren, X. Meng, J. Ren, F. Tang, RSC Adv. 6 (2016) 92839-92844. DOI:10.1039/C6RA21624B |
| [17] |
W. Zhang, Y. Li, H. Zhang, X. Zhou, X. Zhong, Inorg. Chem. 50 (2011) 10432-10438. DOI:10.1021/ic201547g |
| [18] |
C. Xu, R. Zhou, W. He, et al., Anal. Chem. 86 (2014) 3279-3283. DOI:10.1021/ac404244v |
| [19] |
D. Tang, J. Zhang, R. Zhou, et al., Nanoscale 10 (2018) 8477-8482. DOI:10.1039/C8NR01355A |
| [20] |
P. Wu, H. Yu, H.F. Wang, X.P. Yan, Anal. Chem. 82 (2010) 1427-1433. DOI:10.1021/ac902531g |
| [21] |
Z. Wang, Y. Zhang, B. Zhang, X. Lu, Talanta 190 (2018) 1-8. DOI:10.1016/j.talanta.2018.07.065 |
| [22] |
L. Zhang, L. Chen, ACS Appl. Mater. Inter. 8 (2016) 16248-16256. DOI:10.1021/acsami.6b04381 |
| [23] |
S. Signorella, C. Palopoli, G. Ledesma, Coordin. Chem. Rev. 365 (2018) 75-102. DOI:10.1016/j.ccr.2018.03.005 |
| [24] |
A. Tovmasyan, S. Carballal, R. Ghazaryan, et al., Inorg. Chem. 53 (2014) 11467-11483. DOI:10.1021/ic501329p |
 2018, Vol. 29
2018, Vol. 29 

