Polymeric nanoparticles have been considered as perspective drug carriers for various hydrophobic drugs, in particular, hydrophobic anticancer drugs [1-3]. Amphiphilic polymers can be synthesized via grafting hydrophilic polymeric segments with hydrophobic segments and spontaneously form core-shell structure nanoparticles or similar micelle aggregates upon undergoing inter- or intramolecular associations between hydrophobic moieties in an aqueous environment.
Carbohydrates are abundant in nature. Most carbohydrates in nature are polysaccharides, the polymers of monosaccharides through condensation reactions. Natural polysaccharides possess some outstanding physicochemical and biological properties, for instance, non-toxicity, biodegradability, and biocompatibility. Moreover, many polysaccharides have bioadhesion owing to forming non-covalent bonds with biological tissues (particularly mucosal and epithelial surfaces), which are advantageous to gain a prolonged biological half-life in the body [4]. Hereby, natural polysaccharide-based nanoparticles have been extensively explored in the past over two decades [5, 6]. In addition, numerous studies have focused on investigating the synthesis and applications of polysaccharide-based self-aggregate nanoparticles as drug delivery systems [4, 6-8]. Their physicochemical properties in terms of morphology, particle size, and surface characteristics are mainly dependent on the degree of substitution (DS) of the hydrophobic segment in amphiphilic polymers. Generally, average particle size tends to decrease as the DS value increases on account of enhanced hydrophobic interactions [9-11]. Accordingly, it is of vital importance to achieve the optimal DS value for a hydrophobic molecule to prepare nanoparticles along with the desired physicochemical properties. In addition, the nanoparticles that enter the body may undergo interaction with proteins [12, 13]. Bovine serum albumin (BSA) is usually served as a model protein due to its medical importance and similar tertiary structures of human serum albumin [14].
In our previous report, a stearic acid (SA)-modified Bletilla striata polysaccharides (BSPs-SA) conjugate has been synthesized through the esterification reaction between the carboxylic group of SA moiety and the hydroxyl groups of BSPs using 4-dimethylaminopyridine (DMAP) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) as catalysts [15]. 1H NMR spectra of BSPs-SA are provided in Fig. S1 (Supporting information). The BSPs-SA conjugates can spontaneously form stable and monodisperse nanoparticles providing with an approximate average particle sizes of 130 nm by intra- and/or intermolecular self-aggregation in aqueous media as well as presenting a pH-responsive property and good biocompatibility [15, 16].
Herein, we furthermore investigated the effects of the DS values of SA moiety on critical aggregation concentration (CAC), particle size, encapsulation efficiency (EE), loading capacity (LC), and drug release behaviors. The interactions between BSPs-SA nanoparticles and BSA were investigated using the fluorescence quenching technique and ultraviolet (UV) spectroscopy observation. Moreover, the docetaxel (DTX) states in nanoparticles were monitored by X-ray diffraction (XRD) and differential scanning calorimetry (DSC) measurements. The assays for reactive oxygen species (ROS) and lactate dehydrogenase (LDH) release were performed to evaluate the cytotoxicity of BSPs-SA nanoparticles. The DS values of SA moiety were calculated by 1H NMR referring to the equation in the literature [15], which exhibited an increasing tendency with the increase of SA amount in the range of 1%–18.5%. The DS values of SA moiety declined as the increase of reaction temperature whereas the DS values of SA increased along with the molar amount increase of EDC and DMAP (mixed catalysts). Furthermore, it was noteworthy that the BSPs-SA conjugates with higher DS values of SA gave rise to precipitation and could not form nanoparticles under aqueous conditions as a result of higher hydrophobicity. Hereby, three kinds of BSPs-SA conjugates (BSPs-SA4.98, BSPs-SA9.31, and BSPs-SA12.94) along with DS values of 4.98%, 9.31%, and 12.94% were synthesized for the further study.
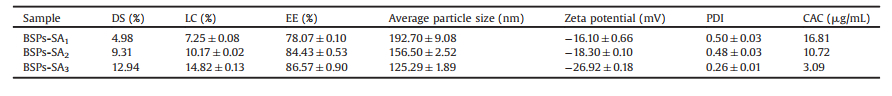
The CAC values of BSPs-SA conjugates were determined by fluorescence spectroscopy utilizing pyrene as a hydrophobic fluorescence probe as the description in the literature [17]. As listed in Table 1, the CAC values of BSPs-SA conjugates decreased when the DS values of SA increased, ascribing to the resultant enhanced hydrophobicity. The CAC values of the BSPs-SA conjugates along with DS values of 4.98%, 9.31%, and 12.94% were 16.81 μg/mL, 10.72 μg/mL and 3.09 μg/mL, respectively.
|
|
Table 1 The effects of the DS values of SA moieties on CAC value, particle size, zeta potential, polydispersity index (PDI), EE and LC of BSPs-SA nanoparticles. Data were offered mean ± S.D. (n = 3). |
BSPs-SA self-aggregated nanoparticles were prepared by dialysis. The zeta potential and particle size of BSPs-SA nanoparticles were conducted on a Malvern Zetasizer Nano-ZS dynamic light scattering (DLS) analyzer. The concentration of the nanoparticles was kept constant at 0.5 mg/mL. The average particle sizes of BSPs-SA nanoparticles increased from 125.29 nm to 192.70 nm as listed in Table 1, remarkably depending on the DS values of SA moiety. As the DS values of SA moiety increased, the particle sizes decreased along with a narrow size distribution, which were consistent with those of the self-aggregates of hydrophobically modified polysaccharides in some previous reports [9-11]. The reason might be a result of the enhanced interaction between the hydrophobic domains in nanoparticles due to the DS increase of SA segment, indicating the formation of more intensive hydrophobic cores [10]. The zeta potential gradually reduced from -16.10 mV to -26.92 mV with the increase of DS values. One possible reason is attributed to BSPs, carrying a negative charge (-12.6 ± 1.99) mV [16]. The second possible reason may be due to SA, which carries negative charge of (-5.43 ± 0.45) mV [16]. The size distribution of nanoparticles exhibited a unimodal size distribution, supplying about 100 nm average diameters and transmission electron microscopy (TEM) image are shown in Figs. S2A and S2B (Supporting information). These results revealed amphiphilic BSPs-SA conjugates could voluntarily form spherical nanoparticles in shape upon self-aggregation behavior. Notably, the particle sizes in TEM image were smaller than those observed in the DLS measurements, which resulted from the drying procedure of sample preparation during the TEM determination [18]. The nanoparticles remained stable even after a week at room temperature (Fig. S3 in Supporting information).
The effects of DS values on EE and LC are also listed in Table 1. The results demonstrated that LC and EE increased from 7.25% ± 0.08% to 14.82% ± 0.13% and 78.07% ± 0.10% to 86.57% ± 0.90% along with the increase of DS values from 4.98% to 12.94%, respectively. The improvement of EE and LC is owing to more hydrophobic sites available for interaction with DTX in the inner cores of self-aggregated nanoparticles when more SA segments were grafted on the hydrophilic chain of BSPs. [19].
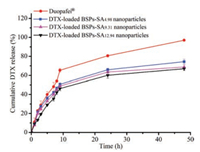
The cumulative release amounts of DTX from BSPs-SA nanoparticles with different DS values of SA are shown in Fig. 1. The release rate of Duopafei® (DTX marketed commercial product) was much more rapid than those of DTX in BSPs-SA nanoparticles. In addition, the cumulative release percentage of DTX in the BSPs-SA nanoparticles were closely related to the DS values of SA, exhibiting a slight decrease with the increase of DS value. The cumulative release amounts of DTX from three BSPs-SA nanoparticles were 74.34%, 68.77% and 66.93% within 48 h, respectively. The phenomenon may be attributed to the strengthened interaction between DTX and hydrophobic domains in nanoparticles because of the increase amount of SA segment [20], restricting DTX release from nanoparticles. Taken together, the BSPs-SA conjugates with the DS value of 12.94% (BSPs-SA12.94) were used to prepare DTX-loaded BSPs-SA nanoparticles for the further experiments.
|
Download:
|
| Fig. 1. In vitro release profiles of DTX from Duopafei® and DTX-loaded BSPs-SA nanoparticles with different DS values of SA moiety in pH 7.4 phosphate buffer saline containing 0.2% of Tween 80 at 37 ± 0.5 ℃. Results were expressed as mean ± S.D. (n = 3). | |
The states of DTX in BSPs-SA nanoparticles were analyzed by XRD and DSC. The results are presented in Fig. S4 (Supporting information). In Fig. S4A, BSPs showed a broad peak at 21.12°. BSPs-SA conjugates appeared a broad peak of 19.18°. The peak difference between BSPs and BSPs-SA conjugates implied the formation of a new substance. Nine typical crystal peaks of DTX emerged at the diffraction peak of 4.66°, 5.38°, 8.02°, 10.12°, 11.28°, 12.56°, 14.06°, 16.96°, and 23.18°. Numerous small peaks were found in the range of 24°–40°. Surprisingly, DTX-loaded BSPs-SA nanoparticles only showed a single diffraction peak of 19.18°. The peak of BSPs-SA conjugates and all typical crystal peaks of DTX disappeared. DSC curves for DTX-loaded BSPs-SA nanoparticles are shown in Fig. S4B. BSPs-SA conjugates showed two endothermic peaks at 175 ℃ and 300 ℃, respectively. DTX displayed two endothermic peaks of 60 ℃ and 225 ℃ but the endothermic peaks of DTX in BSPs-SA nanoparticles disappeared. All the above results implied that DTX was incorporated into in BSPs-SA nanoparticles.
The information about the interactions of BSPs-SA nanoparticles with BSA is shown in Fig. 2. The UV spectra of pure BSA solution, the complex between BSA and BSPs-SA nanoparticles are presented in Fig. 2A. BSA exhibited a strong absorption at 220 nm and a weak absorption at 278 nm. Compared with the spectra of pure BSA solution, the absorbance values at 220 nm and 278 nm increased as the concentrations of BSPs-SA nanoparticles increased. Although the absorption peak at 220 nm showed a slight shift, the absorption peak at 278 nm did not display any shift. This phenomenon implied that BSPs-SA nanoparticles slightly disturbed the microenvironment of protein and so lead to the rearrangement of its tertiary structure, showing a shift of the absorption bands. However, the secondary structure of BSA was not found to be unfolded.

|
Download:
|
| Fig. 2. The UV spectra (A) and fluorescence spectra (B) of BSA in a series of concentrations of BSPs-SA nanoparticles in deionized water (T = 298 K, λex = 293 nm). | |
To confirm the results of UV study, fluorescence quenching was measured to evaluate the structural change of BSA after incubating with BSPs-SA nanoparticles and the excitation wavelength was set at 293 nm. Free BSA exhibited a fluorescence emission at 340 nm wavelength as shown in Fig. 2B. The fluorescence intensities of BSA showed progressive decreases with the concentrations increase of BSPs-SA nanoparticles, revealing that the fluorescence of BSA was effectively quenched by BSPs-SA nanoparticles. However, a slight blue shift at the maximum emission wavelength was found, indicating that the tertiary structure of BSA is slightly altered during the binding procedure [21]. It was seen that the fluorescence intensity of BSA cut down whereas the UV intensity increased gradually with the concentration increase of BSPs-SA nanoparticles. One possible reason is that the UV peaks and fluorescence peaks are produced by the different residues of BSA. The UV peak is a result of tryptophan (Trp) and tyrosine (Tyr) in BAS, but the fluorescence peak is dependent on Trp residues in BSA [22].
The fluorescence quenching of BSA can occur by dynamic quenching or static quenching. Quenching rate constant can be achieved on the basis of the Stern-Volmer equation [9]. The value of quenching rate constant (kq, 7.33 × 1013 L mol-1 s-1) was higher than the limiting diffusion rate constant (2.0 × 1010 L mol-1 s-1), which suggested that the quenching of BSA with BSPs-SA nanoparticles was static quenching (Fig. S5A in Supporting information). Generally, ligands can bind reversibly to BSA [23]. The kb value was calculated to evaluate the affinity intensity of BSA and BSPs-SA nanoparticles. The values of binding constant (kb) and binding site (Fig. S5B in Supporting information) were calculated to be 2.80 × 106 L/mol and 1.107. The value of the binding site was close to 1, which demonstrated that one binding site was available between BSA and BSPs-SA nanoparticles. The kb results illustrated that they possessed a strong binding affinity.
We investigated the cytotoxicity of nanoparticles against 4T1 cells using MTT assay and the results are shown in Fig. S6A (Supporting information). The cell proliferation of DTX-loaded BSPs-SA nanoparticles on 4T1 tumors cells was more potent than that of Duopafei® (P < 0.05) and presented a close concentrationdependent cytotoxicity when equivalent DTX concentration increased from 0.0005 μg/mL to 0.5 μg/mL. One possible reason is that DTX-loaded BSPs-SA nanoparticles can be greatly uptaken by enhanced permeability and retention (EPR) effects [24], resulting in incremental intracellular accumulation of the drug. Another probable reason is that the sustained release behavior of DTX from DTX-loaded BSPs-SA nanoparticles leads to higher efficacy.
To further study the cytotoxic effectsof DTX-loaded BSPs-SA nanoparticles, 4T1 tumor cells were treated with equal concentrations (2 μg/mL) of Duopafei®, BSPs-SA nanoparticles, and DTXloaded BSPs-SA nanoparticles. Cells were harvested in a series of time points and the levels of ROS and LDH were analyzed using ROS and LDH assay kits according to the manufacturer's instructions, respectively. As shown in Fig. S6B (Supporting information), the ROS production exhibited a time-dependent increase. DTX induced an obvious increase of ROS production in comparison to that of the normal control group. LDH levels in extracellular environment of 4T1 cells subjected to Duopafei® and DTX-loaded BSPs-SA nanoparticles were (359.71 ± 6.58 U/L) and (403.5 ± 3.03 U/L), which were 1.53- and 1.71-fold higher compared with the control group (234.74 ± 8.13 U/L), respectively (Fig. S6C in Supporting information). More ROS generation and LDH release were beneficial to induce more cell apoptosis and inhibited the cell proliferation of tumor cells.
In summary, the different CAC values and the hydrophobicity of BSPs-SA conjugates could be altered through grafting different amounts of SA moiety on BSPs. The resultant desired BSPs-SA nanoparticles could be acquired through spontaneously assemble nano-structure particles in aqueous conditions. BSPs-SA nanoparticles exhibited a strong binding to BAS, but could not alter its conformation. BSPs-based self-aggregated nanoparticle may become a promising nanocarrier for anticancer drugs.
AcknowledgmentThe research is supported by Health and Family Planning Commission of Jilin Province (No. 2017J056).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.12.004.
| [1] |
X. Tang, L. Tan, K. Shi, et al., Acta Pharm. Sin. B 8 (2018) 587-601. |
| [2] |
W. Li, J. Peng, Q. Yang, et al., Biomater. Sci. 6 (2018) 1201-1216. DOI:10.1039/C8BM00096D |
| [3] |
Y. Zhang, Y. Yang, K. Tang, et al., J. Appl. Polym. Sci. 107 (2008) 891-897. DOI:10.1002/(ISSN)1097-4628 |
| [4] |
N. Kolishetti, S. Dhar, P.M. Valencia, et al., Proc. Natl. Acad. Sci. U. S. A. 107 (2010) 17939-17944. DOI:10.1073/pnas.1011368107 |
| [5] |
K.Y. Choi, G. Liu, S. Lee, et al., Nanoscale 4 (2012) 330-342. DOI:10.1039/C1NR11277E |
| [6] |
K. Letchford, H. Burt, Eur. J. Pharm. Biopharm. 65 (2007) 259-269. DOI:10.1016/j.ejpb.2006.11.009 |
| [7] |
G. Saravanakumar, D.G. Jo, J.H. Park, Curr. Med. Chem. 19 (2012) 3212-3229. DOI:10.2174/092986712800784658 |
| [8] |
T. Thambi, J.H. Park, J. Biomed. Nanotechnol. 10 (2014) 1841-1862. DOI:10.1166/jbn.2014.1977 |
| [9] |
Y. Wang, Q. Jiang, L.R. Liu, et al., Polymers 48 (2007) 4135-4142. DOI:10.1016/j.polymer.2007.05.034 |
| [10] |
S. Kwon, J.H. Park, H. Chung, et al., Langmuir 19 (2003) 10188-10193. DOI:10.1021/la0350608 |
| [11] |
Q. Xu, X. Yuan, J. Chang, J. Appl. Polym. Sci. 95 (2005) 487-493. DOI:10.1002/(ISSN)1097-4628 |
| [12] |
K. Akiyoshi, T. Nishikawa, Y. Mitsui, et al., Colloids Surf. A 112 (1996) 91-95. DOI:10.1016/0927-7757(96)03560-1 |
| [13] |
S.H.D.P. Lacerda, J.J. Park, C. Meuse, et al., ACS Nano 4 (2010) 365-379. DOI:10.1021/nn9011187 |
| [14] |
X. He, D.C. Carter, Nature 358 (1992) 209-215. DOI:10.1038/358209a0 |
| [15] |
Q. Guan, D. Sun, G. Zhang, et al., Molecules 21 (2016) 1641. DOI:10.3390/molecules21121641 |
| [16] |
L. Zhao, D. Sun, H. Lu, et al., J. Pharm. Pharmacol. 70 (2018) 797-807. DOI:10.1111/jphp.2018.70.issue-6 |
| [17] |
T.E.A. Frizon, Y.M.S. Micheletto, J.L. Westrup, et al., Colloids Surf. B 133 (2015) 323-330. DOI:10.1016/j.colsurfb.2015.06.035 |
| [18] |
H. Du, X. Yang, X. Pang, et al., Carbohydr. Polym. 111 (2014) 753-761. DOI:10.1016/j.carbpol.2014.04.095 |
| [19] |
T.H. Tran, C.T. Nguyen, L.G. Fajardo, et al., Biomacromolecules 15 (2014) 4363-4375. DOI:10.1021/bm5013822 |
| [20] |
X. Shi, Y. Du, J. Yang, et al., J. Appl. Polym. Sci. 100 (2006) 4689-4696. DOI:10.1002/(ISSN)1097-4628 |
| [21] |
G. Li, J. Huang, T. Chen, et al., Carbohydr. Polym. 176 (2017) 75-82. DOI:10.1016/j.carbpol.2017.08.068 |
| [22] |
A. Zhu, L. Yuan, T. Chen, et al., Carbohydr. Polym. 69 (2007) 363-370. DOI:10.1016/j.carbpol.2006.11.023 |
| [23] |
Y. Rahman, S. Afrin, M. Tabish, Arch. Biochem. Biophys. 652 (2018) 27-37. DOI:10.1016/j.abb.2018.06.005 |
| [24] |
Y. Zhang, T. Sun, C. Jiang, Acta Pharm. Sin. B 8 (2018) 34-50. DOI:10.1016/j.apsb.2017.11.005 |
 2018, Vol. 29
2018, Vol. 29