b State Key Laboratory of Bioelectronics, Southeast University, Nanjing 210096, China
In recent years, cancer has become the second leading cause of human death. And the early diagnosis of cancer is critical to the survival of patients and the successful prognosis of the disease [1-4]. Tumor markers are molecules present in blood or tissue and their levels are directly related to the state of cancer. Carcinoembryonic antigen (CEA) [5, 6], as a kind of broad-spectrum tumor marker, directly associates with some malignant tumors including colorectal adenocarcinoma, breast, lung, and ovarian cancer, etc. [7-10]. Thus, developing rapid, sensitive, effective, and convenient detection methods is extremely critical for early diagnosis of CEA. In the past decades, various immunoassays were developed to detect CEA, which including enzyme-linked immunosorbent assay (ELISA) [11], colorimetric immunoassay [12], fluorescent immunoassay [13-17], chemiluminescent immunoassay [18], and electrochemical immunoassay [19]. Compared with the electrochemical immunoassay, however, other methods present some shortcomings of complex and time-consuming processes and environmental contamination [20-22]. Therefore, electrochemical methods are widely used for immunoassays due to the advantages of higher sensitivity, good selectivity, excellent stability, point-of-care detection, and convenient procedures [23-34].
In order to increase the sensitivity of analytical methods, nanomaterials have been introduced into the immunoassays due to their unique physicochemical properties. The gold nanoparticles (Au NPs) [35-41], as a kind of typical nanomaterial, possess high specific surface area, good biocompatibility, excellent electron transfer ability, and good chemical stability, and are widely used in the electrochemical immunoassay. Zhou et al. [42] developed an ultrasensitive label-free immunosensor using protein A/Au NPs functionalized gold electrode as a platform for CEA detection. In their case, the Au NPs not only could enhance the electron transfer but also show the nature of well connected with protein. Li et al. [43] synthesized the Au-F127 nanospheres as a signal amplifier for effective detection of CEA. With the development of silver stain, the gold-silver core-shell (Ag@Au) [44-46] structure was developed and confirmed to have higher electrochemical sensitivity. Mia et al. [47] reported a novel immunoassay based on Ag@Au as a label for ultrasensitive CEA detection in serum samples. And the biosensor displayed properties of selectivity, sensitivity, and simple procedures.
Polythion (PTh) [48] is used as an electrochemical tag for the sensitive detection of proteins in higher isoelectric points. PTh is not only compatible with proteins and electrodes in the electrochemical redox process but also has a strong ability to transfer electrons [49, 50]. Cai et al. [51] fabricated a ratiometric electrochemical biosensor adopting PTh-Au film as an amplification label and Au NPs as a signal enhancer for detecting CEA. Therefore results exhibited that the sensitivity of the immunosensor was significantly amplified due to the synergistic effect of PTh and Au NPs, which suggested that the PTh had satisfied nature of conductivity and biocompatibility.
Herein, a novel electrochemical immunosensor is fabricated using Au NPs-labeled PTh film decorated glassy carbon electrode (GCE) as sensing platform and Ag@Au-connected secondary antibodies (Ag@Au-Ab2) as a tag for detection of CEA. The immunosensor will perform a high sensitivity, excellent selectivity, and good stability for CEA detection.
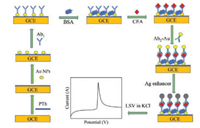
The applied instruments and materials including the synthesis of gold nanoparticles and preparation of secondary antibody (Ab2) labeled with Au NPs in this paper can be found in Supporting information. Fabrication of the immunosensor was shown as follows (Scheme 1). Before electrode modification, the GCE was polished by 0.05 μm alumina slurry, followed by sonicating in ethanol and distilled water for 4 min, respectively [52]. And then, the GCE was placed in 5 mmol/L Thi containing PBS (10 mmol/L, pH 6.8) to electrodeposite at 1.5 V for 15 min, followed by cyclic voltammetry (CV) scanning for 20 cycles at 50 mV/s in the range of -0.6~0.6 V to form PTh film. After washing by distilled water, 10 μL of Au NPs were dropped onto the GCE surface and dried at room temperature. Subsequently, the modified GCE was immersed in 10 μL of 10 μg/mL Ab1 for self-assembles at 4 ℃ overnight under 100% humidity, followed by washing with PBST to remove excess Ab1. After that, 10 μL of blocking buffer was applied to the functionalized GCE for 1 h to block uncovered active sites. Followed by washing with PBST, 10 μL of 120 ng/mL CEA standard sample was incubated on GCE surface for 1 h at room temperature, and gently rinsing with PBST. Later, 10 μL of 10 μg/mL Ab2-Au was dropped on the electrode and incubated for 1 h, rinsed by PBST to avoid non-specific adsorption. Then, 10 μL of an equal volume of 14 mmol/L AgNO3 containing citrate buffer (0.1 mol/L, pH 3.5) and 45 mmol/L HQN mixture were rapidly added on the sensor and reacted at 4 ℃ in the dark for 10 min. After thoroughly rinsing, the sensor was performed by LSV at -0.2~0.3 V in 1 mol/L KCl for detecting silver anodic stripping peak.
|
Download:
|
| Scheme 1. Schematic principle of the electrochemical immunosensor based on gold-label silver-stain for CEA detection. | |
The stepwise modification process of the electrode using [Fe(CN)6]4-/3- as a probe was showed by differential pulse voltammetry (DPV) in the electrolyte (Fig. 1). Compared with bare GCE (curve b), the PTh-Au modified GCE (curve a) displayed obviously stronger current response at 0.184 V (vs. Ag/AgCl) due to the excellent conductivity of Au NPs and PTh. However, after the modification of Ab1 (curve c), the current response is dramatically reduced because of the formation of the electron blocking layer on the electrode surface, suggesting the successful combination of Ab1 on the electrode. And further current decrease appeared after blocking buffer was dropped on the electrode surface, indicating the BSA (curve d) had successfully blocked the excess active sites. When CEA (curve e) was adsorbed on the sensor, the peak current continued to decrease. Because the protein barrier of CEA hindered the transfer of electrons on the electrode surface, which indicated the efficient immobilization of CEA. Finally, followed by the adsorption of Ab2-Au (curve f), the current response dropped. The reason for the phenomenon is that immunoreaction of Ab2-Au with CEA made the larger protein barrier, and further hindered electron transfer on the electrode surface. According to the data, the change of current response proved that PTh-Au, Ab1, BSA, CEA, and Ab2-Au were continuously assembled to the GCE surface.

|
Download:
|
| Fig. 1. DPV response of stepwise modification process of the electrode in 10 mmol/L [Fe(CN)6]4-/3- (containing 0.1 mol/L KCl)-PBS (pH 6.8): (a) Au-PTh/GCE; (b) bare GCE; (c) Ab1/Au-PTh/GCE; (d) BSA/Ab1/Au-PTh/GCE; (e) CEA/BSA/Ab1/Au-PTh/GCE; (f) Au-Ab2/CEA/BSA /Ab1/Au-PTh/GCE. | |
In order to obtain the optimum current response of the immunosensor, some important factors influencing the electrochemical signal were investigated, such as the incubation time of antibody and antigen, the concentration of silver nitrate, and the silver deposition time.
To study the optimal conditions of the proposed immunosensor, the incubation time of immunoreaction between (1) Ab1 and CEA and (2) CEA and Ab2-Au were investigated. The 10 μL of 120 ng/mL CEA was dropped on the immunosensor surface and incubated from 10 min to 60 min. the current-time dependence plots shown in Fig. S2 (Supporting information), the DPV response continuously dropped prior to 40 min and did not significantly change from 40 min to 60 min, which suggested that the amount of CEA was captured and saturated on the sensing interface. Thus, the optimum incubation time of Ab1 and CEA conjugation was 40 min. After that, the functionalized sensors were immersed in 10 μL of 10 μg/mL Ab2-Au solution from 10 min to 60 min. As shown in Fig. S3 (Supporting information), the peak current of DPV decreased with the accumulation of incubation time, and then tended to level off at 50 min, which revealed that the conjugation of CEA and Ab2-Au was saturated under the incubation time at 50 min. Thus, 50 min is the best incubation time of CEA and Ab2-Au for immunoreaction.
For further signal enhancement, Ag@Au was selected as an effective detection label of the immunosensor. Herein, the influences of silver nitrate concentration and silver deposition time on the anodic stripping current of the sensor were examined. As shown in Fig. S4 (Supporting information, curve a), a significant increase in the stripping voltammetric responses with the increase in silver nitrate concentration in the range of 2-14 mmol/L was presented and it reached a stable value after silver nitrate concentration of 14 mmol/L. To avoid background current interference, the control group without CEA was designed (Fig. S4, curve b), which indicated the existence of background signals and it gradually increased with the concentration of silver nitrate escalation. Therefore, the Ag+ concentration with the highest signal difference between samples with and without CEA was applied to determine the optimum concentration at 14 mmol/L. Later, the silver deposition time was also tested within 10 μL of mixture containing 14 mmol/L AgNO3 and 45 mmol/L HQN. According to Fig. S5 (Supporting information), the stripping current increased rapidly and approached saturation in the first 10 min. Thus, 10 min was the optimal silver deposition time in the further assay.
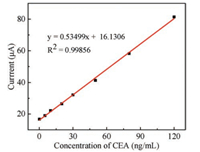
Under optimal conditions, LSV measurement was used to analyze the performance of the immunosensor. Fig. 2 showed the typical LSV responses with increasing concentrations of CEA (0.1, 5, 10, 20, 30, 50, 80, 120 ng/mL, from bottom to top) in 1 mol/L KCl. As shown in Fig. 3, the stripping current exhibited a wide linear calibration curve, when the CEA concentrations increased from 0.1 ng/mL to 120 ng/mL. The linear regression equation was adjusted to y (μA) = 0.53499x (ng/mL) + 16.1306 (R2 = 0.99856) with the detection limit as low as 0.055 ng/mL (S/N = 3).

|
Download:
|
| Fig. 2. LSV responses of the immunosensor to CEA at concentrations of (a) 0.1 ng/mL, (b) 5 ng/mL, (c) 10 ng/mL, (d) 20 ng/mL, (e) 30 ng/mL, (f) 50 ng/mL, (g) 80 ng/mL, (h) 120 ng/mL. | |
|
Download:
|
| Fig. 3. The linear calibration of the immunosensor for CEA detection from 0.1 ng/mL to 120 ng/mL. | |
The reproducibility of the immunosensor was evaluated by LSV measurement, and five equal immunosensors [36] were fabricated for the detection of CEA (10 μL, 80 ng/mL). The peak currents were 58.84 μA, 56.65 μA, 59.17 μA, 58.2 μA, 58.22 μA, respectively. According to calculation, the relative standard deviation (RSD) of the five immunosensors was 6.45%, indicating an excellent reproducibility of the immunosensor.
The stability of the immunosensor was also considered by detecting the LSV signal after fifteen days of storage (at 4 ℃). Compared with the initial value of the stripping current, the current signal approximately reduced 3.16% (less than 5%), which revealed that the stability of the immunosensor was good.
To further assess the selectivity of the immunosensor, interference experiment was performed using AFP, BSA, AA, and glucose, respectively. The 10 μL of 35 ng/mL CEA solution was mixed with 100 ng/mL of interfering substances and measured by the LSV assay. As shown in Fig. S6 (Supporting information), the current variation deriving from the interfering substances ranged from 0.9%-4.8% (less than 5%), indicating a high selectivity of the proposed immunosensor.
In summary, a novel enzyme-free electrochemical immunosensor was developed using PTh-Au as the sensing platform and Ag@Au-Ab2 as a tag for sensitively detecting CEA. In this work, the PTh-Au displayed a high conductivity and good biocompatibility for the sensing surface. Moreover, the Ag@Au-Ab2, as a good detection signal amplifier, showed high efficiency and high sensitivity for the CEA detection. The study results exhibited that the square of correlation coefficients were 0.99856 with the wide concentration range of 0.1-120 ng/mL for CEA detection, and the detection limit was 0.055 ng/mL (S/N = 3). Moreover, the immunosensor presented high reproducibility, stability, and selectivity. The result indicated the potential application for the early clinical diagnosis of CEA.
AcknowledgmentsWe acknowledge the financial supports of the National Natural Science Foundation of China (Nos. 61471168, 61571187), China Postdoctoral Science Foundation (No. 2016T90403), Postdoctoral Science Foundation of Jiangsu Province (No.1601021A), the Natural Science Foundation of Hunan Province (No. 2017JJ209), and Hunan Key Research Project (No. 2017SK2174).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.11.030.
| [1] |
I.E. Tothill, Semin. Cell Dev. Biol. 20 (2009) 55-62. DOI:10.1016/j.semcdb.2009.01.015 |
| [2] |
C. Sun, L. Ma, Q. Qian, et al., Analyst 139 (2014) 4216-4222. DOI:10.1039/C4AN00479E |
| [3] |
Y.S. Gao, J.K. Xu, L.M. Lu, et al., RSC Adv. 5 (2015) 86910-86918. DOI:10.1039/C5RA16618G |
| [4] |
S.Y. Xue, H.Y. Yi, P. Jing, W.J. Xu, RSC Adv. 5 (2015) 77454-77459. DOI:10.1039/C5RA15038H |
| [5] |
M.J. Engelen, H.W. de Bruijn, H. Hollema, et al., Gynecol Oncol. 78 (2000) 16-20. DOI:10.1006/gyno.2000.5811 |
| [6] |
Y.X. Lai, Y. Deng, G.J. Yang, et al., J. Biomed. Nanotechnol. 14 (2018) 1688-1694. DOI:10.1166/jbn.2018.2617 |
| [7] |
K.J. Huang, D.J. Niu, W.Z. Xie, W. Wang, Anal. Chim. Acta 659 (2010) 102-108. DOI:10.1016/j.aca.2009.11.023 |
| [8] |
S. Ge, F. Yu, L. Ge, et al., Analyst 137 (2012) 4727-4733. DOI:10.1039/c2an35967g |
| [9] |
F. Naghibalhossaini, P. Ebadi, Cancer Lett. 234 (2006) 158-167. DOI:10.1016/j.canlet.2005.03.028 |
| [10] |
J.D. Wulfkuhle, L.A. Liotta, E.F. Petricoin, Nat. Rev. Cancer 3 (2003) 267-275. DOI:10.1038/nrc1043 |
| [11] |
W.J. Zhou, J. Su, Y.Q. Chai, R. Yuan, Y. Xiang, Biosens. Bioelectron. 53 (2014) 494-498. DOI:10.1016/j.bios.2013.10.020 |
| [12] |
C. Luo, W. Wen, F. Lin, et al., RSC Adv. 5 (2015) 10994-10999. DOI:10.1039/C4RA14833A |
| [13] |
Q. Yu, X. Wang, Y. Duan, Anal. Chem. 86 (2014) 1518-1524. DOI:10.1021/ac402973n |
| [14] |
X.L. Ren, J.J. Ge, S. Li, et al., J. Biomed. Nanotechnol. 13 (2017) 1425-1434. DOI:10.1166/jbn.2017.2432 |
| [15] |
H.W. Yang, M. Liu, H.R. Jiang, et al., J. Biomed. Nanotechnol. 13 (2017) 655-664. DOI:10.1166/jbn.2017.2386 |
| [16] |
Z.Q. Bai, X.L. Ren, Z. Gong, et al., Chin. Chem. Lett. 28 (2017) 1901-1904. DOI:10.1016/j.cclet.2017.05.005 |
| [17] |
T.T. Li, H. Yi, Y. Liu, et al., J. Biomed. Nanotechnol. 14 (2018) 150-160. DOI:10.1166/jbn.2018.2491 |
| [18] |
L.L. Chen, Z.J. Zhang, P. Zhang, X.M. Zhang, A.H. Fu, Sensor. Actuat. B -Chem. 155 (2011) 557-561. DOI:10.1016/j.snb.2011.01.007 |
| [19] |
R. Wang, J.J. Feng, Y.D. Xue, L. Wu, A.J. Wang, et al., Sensor. Actuat. B -Chem. 254 (2018) 1174-1181. DOI:10.1016/j.snb.2017.08.009 |
| [20] |
T. Feng, X. Chen, X. Qiao, et al., Anal. Biochem. 494 (2016) 101-107. DOI:10.1016/j.ab.2015.11.004 |
| [21] |
W. Lu, C. Qian, L. Bi, et al., Biosens. Bioelectron. 53 (2014) 346-354. DOI:10.1016/j.bios.2013.10.007 |
| [22] |
M. Rizwan, S. Elma, S.A. Lim, M.U. Ahmed, Biosens.Bioelectron. 107 (2018) 211-217. DOI:10.1016/j.bios.2018.02.037 |
| [23] |
D. Wu, H. Ma, Y. Zhang, et al., ACS Appl. Mater. Interfaces 7 (2015) 18786-18793. DOI:10.1021/acsami.5b05443 |
| [24] |
Y. Wang, Y. Wang, D. Wu, et al., Sensor. Actuat. B -Chem. 255 (2018) 125-132. DOI:10.1016/j.snb.2017.07.129 |
| [25] |
Y. Yang, Q. Liu, Y. Liu, et al., Biosens. Bioelectron. 90 (2017) 31-38. DOI:10.1016/j.bios.2016.11.029 |
| [26] |
H. Wang, Z. Ma, Microchimica Acta 184 (2017) 1045-1050. DOI:10.1007/s00604-017-2101-y |
| [27] |
Y. Liu, Y.X. Lai, G.J. Yang, et al., J. Biomed. Nanotechnol. 13 (2017) 1253-1259. DOI:10.1166/jbn.2017.2424 |
| [28] |
Y. Huang, J. Tan, L.J. Cui, et al., Biosens. Bioelectron. 102 (2018) 560-567. DOI:10.1016/j.bios.2017.11.037 |
| [29] |
Y.X. Lai, C.X. Zhang, Y. Deng, et al., Chin. Chem. Lett. (2018) 29. DOI:10.1016/j.cclet.2018.07.011 |
| [30] |
Y. Xing, X.Z. Feng, L.P. Zhang, et al., Int. J. Nanomed. 12 (2017) 3171-3179. DOI:10.2147/IJN |
| [31] |
L.L. Cao, C. Fang, R.S. Zeng, et al., Sensor. Actuat. B -Chem. 252 (2017) 44-54. DOI:10.1016/j.snb.2017.05.148 |
| [32] |
Y. Liu, K.K. Liu, H.M. Dong, et al., Nanosci. Nanotechnol. Lett. 8 (2016) 785-790. DOI:10.1166/nnl.2016.2264 |
| [33] |
L.L. Cao, C. Fang, R.S. Zeng, et al., Biosens. Bioelectron. 92 (2017) 87-94. DOI:10.1016/j.bios.2017.02.002 |
| [34] |
Z. Liu, W. Li, G.C. Han, et al., J. Electrochem. Soc. 161 (2014) B75-B80. DOI:10.1149/2.022405jes |
| [35] |
T. Springer, X.C. Song, M.L. Ermini, J. Lamacová, J. Homola, Anal. Bioanal. Chem. 9409 (2017) 4087-4097. |
| [36] |
X.H. Wang, H.S. Peng, W. Yang, Z.D. Ren, Y.A. Liu, Nanosci. Nanotechnol. Lett. 9 (2017) 227-232. DOI:10.1166/nnl.2017.2317 |
| [37] |
S.Y. Pang, N. Sheng, J.P. Wang, et al., J. Biomed. Nanotechnol. 13 (2017) 1178-1209. DOI:10.1166/jbn.2017.2423 |
| [38] |
H.D. Cui, D.H. Hu, J.N. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1391-1398. DOI:10.1016/j.cclet.2016.12.038 |
| [39] |
J. Yang, F.J. Nian, Z.R. Guo, T.T. Bai, G. Peng, Nanosci. Nanotechnol. Lett. 9 (2017) 1491-1496. DOI:10.1166/nnl.2017.2503 |
| [40] |
Y.P. Jia, B.Y. Ma, X.W. Wei, Z.Y. Qian, Chin. Chem. Lett. 28 (2017) 691-702. DOI:10.1016/j.cclet.2017.01.021 |
| [41] |
Y. Li, J. Sun, Q.P. Chen, L. Zhu, Nanosci. Nanotechnol. Lett. 9 (2017) 982-987. DOI:10.1166/nnl.2017.2430 |
| [42] |
J. Zhou, L. Du, L. Zou, et al., Sensor. Actuat. B -Chem. 197 (2014) 220-227. DOI:10.1016/j.snb.2014.02.009 |
| [43] |
J. Li, H. Xie, Y. Liu, et al., Talanta 144 (2015) 404-410. DOI:10.1016/j.talanta.2015.06.065 |
| [44] |
P. Duangkaew, S. Tapaneeyakorn, C. Apiwat, et al., Biosens. Bioelectron. 74 (2015) 673-679. DOI:10.1016/j.bios.2015.07.004 |
| [45] |
K.N. Zhu, Z.Y. Zhu, H.O. Zhou, J.Y. Zhang, S.Y. Liu, Chin. Chem. Lett. 28 (2017) 1276-1284. DOI:10.1016/j.cclet.2017.03.020 |
| [46] |
T. Bai, P. Lu, K. Zhang, et al., J. Biomed. Nanotechnol. 13 (2017) 1178-1209. DOI:10.1166/jbn.2017.2423 |
| [47] |
X. Miao, S. Zou, H. Zhang, L.S. Ling, Sensor. Actuat. B -Chem. 191 (2014) 396-400. DOI:10.1016/j.snb.2013.10.016 |
| [48] |
Y. Sun, Y. Bai, W. Yang, C.Q. Sun, Electrochimica Acta 52 (2007) 7352-7361. DOI:10.1016/j.electacta.2007.06.007 |
| [49] |
C. Yang, Q. Wang, Y. Xiang, R. Yuan, Y.Q. Chai, Sensor. Actuat. B -Chem. 197 (2014) 149-154. DOI:10.1016/j.snb.2014.02.036 |
| [50] |
F.Y. Kong, M.T. Xu, J.J. Xu, H.Y. Chen, Talanta 85 (2011) 2620-2625. DOI:10.1016/j.talanta.2011.08.028 |
| [51] |
X. Cai, S. Weng, R. Guo, et al., Biosens. Bioelectron. 81 (2016) 173-180. DOI:10.1016/j.bios.2016.02.066 |
| [52] |
H. Wang, Z. Ma, Microchimica Acta 184 (2017) 3247-3253. DOI:10.1007/s00604-017-2287-z |
 2018, Vol. 29
2018, Vol. 29 

