In the past few decades, the exploration of polymeric micelles with different functions in cancer therapy remains as a research hot spot [1, 2]. These nanoparticles tend to be enriched in tumor tissue by enhanced permeability and retention (EPR) effect, and thus enhance the utilization rate of drug and avoid the side effect [3, 4]. In order to unload the encapsulated drug when arriving at tumor site, micelles responsive to various stimuli, such as pH [5], redox [6], electric field [7], enzymes [8], have been developed. Among them, pH-sensitive micelles have attracted great attention for drug delivery due to the lower pH value of tumor site compared to normal tissue regardless of tumor type [9].
Poly(β-amino ester) (PAE) is a kind of typical pH-responsive polymers which can transform hydrophobic form to hydrophilic form in weakly acid condition because of the protonation of the tertiary amine groups in the main chain [10]. PAEs with different modification have been universally used to encapsulate various kinds of drugs [5, 11-13]. However, these polymer materials were predominantly built via covalent-connection and in most cases needed complex synthetic procedures to make polymer structure meet the requirement of its self-assembling into micelles. To solve this problem, micellar nanoparticles structured by non-covalent interactions are preferably tried.
In recent years, supramolecular polymers based on noncovalent host-guest molecular interactions have gained increasing attention [14]. The main factors of the inclusion complexation are van der Waals force and hydrophobic interactions, and sometimes hydrogen bonding and steric effects also play a role [15]. The dynamic and reversible characteristics of host-guest interactions make it easier to fabricate polymers with various structures and functions [16-19].
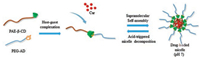
However, only a few studies combined host-guest recognition with stimulating responsive polymers. Herein, we report a pHresponsive supramolecular micelle consisting of a hydrophobic PAE core and a hydrophilic PEG shell based on the host-guest interaction between β-cyclodextrin (β-CD) and adamantane (AD) for drug delivery (Scheme 1). β-CD possesses a hydrophobic central cavity suitable for the inclusion of various guest molecules. The size of AD is almost completely adapted to the cavity of β-CD, and once complexed, AD fits deep in the β-CD cavity [20]. The association constant Ka is as high as 1 ×105 mol-1 [21]. The host molecule β-cyclodextrin-contained poly(β-amino ester) (PAE-β-CD) and guest molecule adamantyl-terminated poly(ethylene glycol) (PEG-AD) were synthesized. They formed host-guest inclusion complexes and further self-assemble into micelles. Curcumin (Cur) was chosen as the model drug to be loaded into micelles for its anticancer effect [22]. The pH responsiveness of supramolecular micelle and its drug loading ability were investigated, and the pH-triggered drug release behavior was also examined. Besides, the antitumor efficiency was evaluated both in vitro and in vivo.
|
Download:
|
| Scheme 1. Schematic illustration of Cur-loaded supramolecular micelles self-assembled from PAE-β-CD and PEG-AD via host-guest complexation | |
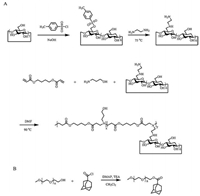
Firstly, PAE-β-CD was synthesized (Scheme 2A). Mono(6-(2- aminoethyl)amino-6-deoxy)-β-cyclodextrin (β-CD-EDA) was obtained according to the procedure reported elsewhere with a few modifications [16]. Then, 3-amino-1-propanol (3-AP) (1.50 g, 20 mmol), butanediol diacrylate (4-BD) (4.36 g, 22 mmol) and β-CD-EDA (2.35 g, 2 mmol) were dissolved in 15 mL of anhydrous N, N-dimethylformamide (DMF) in a flask, and the reaction was incubated at 90 ℃ for 12 h under a nitrogen atmosphere. After cooled to the room temperature, the final product was precipitated in cold diethyl ether for three times and subsequently dried under vacuum to get PAE-β-CD.
|
Download:
|
| Scheme 2. Synthesis routes of (A) PAE-β-CD and (B) PEG-AD | |
PEG-AD was synthesized by the reaction of MPEG with 1- adamantanecarbonyl chloride [23] (Scheme 2B). MPEG (2 g, 1 mmol), 4-dimethylamino-pyridine (DMAP) (12.2 mg, 0.1 mmol) and triethylamine (TEA) (0.3 mL) were dissolved in anhydrous dichloromethane (DCM) in a flask and then cooled in an ice bath for 30 min. 1-Adamantanecarbonyl chloride (0.4 g, 2 mmol) dissolved in 10 mL of anhydrous DCM was added dropwise under nitrogen. The system was stirred at 0 ℃ for 2 h, followed by further reaction for 24 h. Then, the mixture was filtered to remove the insoluble TEA hydrochloride, and the filtrate was precipitated with an excess amount of diethyl ether for three times to get a white solid. Subsequently, this solid was dried under vacuum to obtain the final product PEG-AD.
The structures of the β-CD-EDA and PAE-β-CD were examined by 1H nuclear magnetic resonance (1H NMR) spectra. As shown in Fig. S1 (Supporting information), all the protons of β-CD-EDA could be observed [16]. After synthesized with 3-AP and 4-BD, the main peaks of β-CD still existed. In Fig. S2 (Supporting information), the new peaks at δ 1.48, 1.62, 2.38, 2.65 and 4.0 demonstrated the successful synthesis of PAE-β-CD. Peak a at 4.8 of β-CD and peak b' at 4.0 of PAE were selected to calculate the ratio of two chain units. From gel permeation chromatography (GPC) analysis, the number average molecular weight and the polydispersity index (PDI) of PAE-β-CD were determined to be 3869 Da and 1.85. Integrating GPC data and 1H NMR [11], x and y came out to be 9 and 1, respectively, demonstrating that PAE-β-CD contains one β-CD in its structure. Fourier transform infrared (FT-IR) spectra of PAE-β-CD and β-CDEDA were compared. As shown in Fig. S3 (Supporting information), the FT-IR spectrum of β-CD-EDA exhibits the O-H stretching vibrations at 3380 cm-1, O-C-O stretching vibrations at 1030 cm-1 and the ring vibrations at 578, 708, 758, and 947 cm-1 [24, 25]. In the spectrum of PAE-β-CD, the characteristic peaks of β-CD still existed. Furthermore, a peak at 1730 cm-1 appeared, which was assigned to C=O stretching vibrations, supporting the former conclusion of 1H NMR. Besides, the pHresponsive property of PAE-β-CD was confirmed by acid-base titration and optical measurement method (Fig. S5 in Supporting information), PAE-β-CD had an obvious buffer region and exhibited evident pH responsiveness. The pKb of the polymer was about 6.9.
The structure of PEG-AD was characterized by 1H NMR. As shown in Fig. S4 (Supporting information), the peaks at δ 1.6~2.1 corresponded to the adamantyl moieties while the peaks at δ 3.65 and 3.38 were attributed to the protons of methylene and methoxy groups on PEG respectively, and peak at 4.20 was ascribed to the methylene directly linked with the ester bond. By comparing the area of peaks at δ 3.38 and 4.20, the degree of end adamantylmodification exceeded 95%.
PAE-β-CD and PEG-AD could form inclusion complexes via the host-guest interaction between β-CD and adamantane. To examine this interaction between polymers, the freeze-dried blank micelle was dissolved in D2O, and the two-dimensional nuclear overhauser effect spectroscopy (2D-NOESY) NMR spectra were recorded. As shown in Fig. 1, the correlations between protons on C3 and C5 of β-CD(δ3.5~3.8) and protons of adamantane (δ 1.6~2.0) were clearly observed, indicating that the supramolecular polymer was successfully obtained.

|
Download:
|
| Fig. 1. 2D 1H NOESY spectra of supramolecular polymer formed by PAE-β-CD and PEG-AD in D2O | |
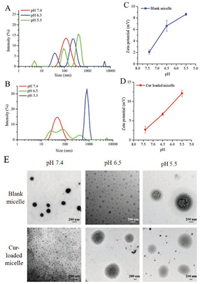
After the synthesis and characterization of materials, the supramolecular micelles were prepared using a dialysis method. Typically, PAE-β-CD and PEG-AD were dissolved in DMF, followed by sonication for 30 min. The solution was then added dropwise in four volumes of deionized water under vigorous stirring. The mixture was transferred to a dialysis bag (MWCO: 8–14 kDa) and dialyzed against water for 5 h. After that, the solution was filtered and freeze-dried. The size distribution and zeta potential of micelles were measured by dynamic light scattering (DLS). As shown in Fig. 2A, at physiological pH 7.4, the size of blank micelles was about 121.1 nm with a narrow distribution (PDI, 0.161). The morphology of blank micelles was observed by transmission electron microscope (TEM) (Fig. 2E), which were solid spherical with the particle size less than 200 nm, similar to the result obtained by DLS. When the micelles were incubated at pH 6.5, the size distribution of blank micelles measured by DLS obviously changed, most of the micelles had a larger size and the distribution of micelles became wider sharply. When the pH value was 5.5 (similar to endosomal compartments), the particle size of blank micelles was further enlarged and reached 500 nm. In the TEM images of blank micelles (Fig. 2E), at pH 6.5, the size of the blank micelles became noticeably uneven and the color became light. When the pH was lowered to 5.5, the blank micelles became larger and many pores appeared in the micelles, indicating that the micelles were obviously cracked under this environment. This was because the PAE chain contained a large amount of tertiary amine groups. After the pH was lowered, these tertiary amine groups were protonated, and the PAE segment changed from hydrophobic to hydrophilic [3], resulting in loose or even cleavage of the micelle structure, which made the particle size become larger. Furthermore, when the pH dropped from 7.4 to 5.5, the zeta potential of the blank micelles increased from 2.09 mV to 8.65 mV (Fig. 2C), which also demonstrated the protonation process of the PAE chain.
|
Download:
|
| Fig. 2. Size distribution of (A) blank micelles and (B) Cur-loaded micelles at pH 7.4, 6.5, 5.5. Zeta potential of (C) blank micelles and (D) Cur-loaded micelles at pH 7.4, 6.5, 5.5. (E) TEM images of blank micelles and Cur-loaded micelles at pH 7.4, 6.5, 5.5 | |
Cur-loaded supramolecular micelles were prepared with the similar method as the blank micelles except that Cur was added and dissolved into the sonicated solution. The loading content (LC) could reach 6.16%, and the encapsulation efficiency (EE) was 85.5%. After encapsulating Cur, the particle size of drug-loaded micelle was significantly reduced. As shown in Fig. 2B, at pH 7.4, the size measured by DLS was 48.2 nm, and the PDI was about 0.126. The reason of this phenomenon may be the interaction between the polymer material and the drug [26], which made the hydrophobic core denser. Similar phenomena have been reported in the previous literature [27]. When the pH was lowered, the size of the Cur-loaded micelles had similar changes to the blank micelles. It can be seen from DLS (Fig. 2B) and TEM (Fig. 2E) that the particle size of the micelle became large and the micelles were uneven. At pH 5.5, the particle size of the Cur-loaded micelles increased to approximately 800 nm, indicating that the Cur-loaded micelles significantly decomposed at this pH value. The zeta potential of the Cur-loaded micelles increased from 2.68 mV to 12.1 mV (Fig. 2D), which had the same trend as changes in blank micelles.
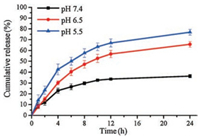
The drug release behavior of Cur-loaded micelles was examined at three pH values of 5.5, 6.5, and 7.4. Fig. 3 shows that the release of Cur from the micelles was varied clearly at different pH circumstance. At pH 7.4, the cumulative release of Cur within 24 h was less than 40%. When the pH was 6.5, the release of Cur increased significantly, and about 65% of the drug was released within 24 h. When the pH was set to 5.5, the release amount was further increased. The cumulative release of Cur exceeded 40% in 4 h and about 80% of Cur could be released in 24 h. The result indicated that the Cur-loaded micelles had obvious pH responsiveness due to the transition of PAE from hydrophobic to hydrophilic as discussed above. The tertiary amine groups on the PAE segments were protonated in acid condition, and the hydrophobic core formed by PAE became hydrophilic, which led to the collapse of the Cur-loaded micelles. Thus, the Cur encapsulated in the micelle was released, and the cumulative release rate varies significantly depending on the pH value.
|
Download:
|
| Fig. 3. Release behavior of Cur from Cur-loaded micelle at pH 7.4, 6.5 and 5.5 | |
To evaluate the antitumor efficiency of micelles, a series of cell and animal experiments were carried out. The in vitro toxicity of micelles was determined by the MTT assay. As shown in Fig. S6 (Supporting information), the cell viability did not change substantially with the increase of the blank micelle concentration. Even when at a high micelle concentration (400 μg/mL), the cell viability still remained about 80%. It showed that the blank micelles had low toxicity and had certain biosafety to be used in vivo. The cytotoxicity of Cur-loaded micelle was presented in Fig. S7 (Supporting information), as the concentration of Cur-loaded micelle raised, the cell viability declined obviously. When the concentration of Cur-loaded micelle was increased to 15 μg/mL, the cell viability of S180 decreased to 24.58%. Besides, the 50% inhibitory concentration (IC50) value of Cur-loaded micelle was 5.07μg/mL. The results showed that Cur-loaded micelle had significant in vitro antiproliferation effect.
To confirm the enhanced cellular uptake of Cur caused by micelle encapsulation, the mouse sarcoma 180 (S180) cells were cultured with free Cur and Cur-loaded micelles respectively. Confocal laser scanning microscopy (CLSM) images of cell cultured for 1 h and 4 h were shown in Fig. S8 (Supporting information). S180 cells were stained blue by 40, 6-diamidino-2-phenylindole (DAPI) and Cur emitted green fluorescence. In the cells co-cultured with the drug-loaded micelles, Cur apparently entered the cells. And the amount of Cur entering the cells increased with the prolongation of the culture time. In contrast, the cellular uptake of free Cur was minimal, and after four hours of incubation, the fluorescence of Cur in the cells was extremely weak, indicating that the free Cur could hardly enter the cells.
The in vivo antitumor effect of the different formations was determined using S180 tumor bearing mice. The mice treated with saline served as control group. The blank micelle was used to study the toxicity of carrier in vivo. The mice of experimental groups were treated with two different doses of Cur-loaded micelles. Fig. 4A shows the tumor growth condition during the experiment. The mice treated with high dose of Cur-loaded micelle (Cur 40 mg/kg) showed obviously smaller tumor sizes than other three groups, and the tumor inhibition rate reaches 62.14% when compared with the control group. Mice treated with blank micelles exhibited similar fast growth rate as those treated with saline and low-dose drug treated mice (Cur 20 mg/kg) showed slight therapeutic effects. The photos of collected tumor at the end of treatment showed the same result (Fig. S9 in Supporting information). What is more, no significant differences in body weight were observed between the different groups of mice (Fig. 4B), which indicated that the micelles were safe in vivo. Through the above experimental analysis, it can be known that the Cur-loaded micelles have an acceptable anti-tumor effect at a relatively high dose.

|
Download:
|
| Fig. 4. In vivo anticancer activity. (A) S180 tumor volume growth curves of mice after different treatment (n=5, ** P < 0.01). (B) Body weights of mice after different treatments | |
In summary, we constructed a novel pH sensitive supramolecular micelle based on non-covalent host-guest interaction between β-CD contained PAE and AD-terminated PEG. The supramolecular micelles could effectively encapsulate the anti-cancer drug Cur. The remarkable pH-responsiveness of the micelles was confirmed by pH-triggered drug release behavior and morphological changes at different pH values. Furthermore, both in vitro and in vivo experiments have demonstrated the biosafety and antitumor effects of the Cur-loaded micelles. Overall, this supramolecular nanosystem may have potential as a stimulus responsiveness carrier for hydrophobic drug entrapment and provide new directions for the design and construction of stimuli-responsive supramolecular vectors.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 81473173).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.11.009.
| [1] |
G.C. Yu, W. Yu, L. Shao, et al., Adv. Funct. Mater. 26 (2016) 8999-9008. DOI:10.1002/adfm.v26.48 |
| [2] |
K. Yang, L. Feng, Z. Liu, Adv. Drug Deliv. Rev. 105 (2016) 228-241. DOI:10.1016/j.addr.2016.05.015 |
| [3] |
S. Tang, Q.S. Meng, H.P. Sun, et al., Biomaterials 114 (2017) 44-53. DOI:10.1016/j.biomaterials.2016.06.005 |
| [4] |
C. Gao, F. Tang, G.Y. Gong, et al., Nanoscale 9 (2017) 12533-12542. DOI:10.1039/C7NR03611F |
| [5] |
C. Zhang, T. An, D. Wang, et al., J. Control. Release 226 (2016) 193-204. DOI:10.1016/j.jconrel.2016.02.030 |
| [6] |
D.Q. Chen, G.Q. Zhang, R.M. Li, et al., J. Am. Chem. Soc. 140 (2018) 7373-7376. DOI:10.1021/jacs.7b12025 |
| [7] |
L. Peng, Z.L. Wang, A.C. Feng, et al., Polymer 88 (2016) 112-122. DOI:10.1016/j.polymer.2016.02.023 |
| [8] |
P.D. Thornton, R.J. Mart, R.V. Ulijn, Adv. Mater. 19 (2007) 1252-1256. |
| [9] |
Z. Zhang, J.X. Ding, X.F. Chen, et al., Polym. Chem. 4 (2013) 3265-3271. DOI:10.1039/c3py00141e |
| [10] |
P. Shen, L.Y. Qiu, New J. Chem. 42 (2018) 3593-3601. DOI:10.1039/C7NJ05042A |
| [11] |
Q.N. Bui, Y. Li, M.S. Jang, et al., Macromolecules 48 (2015) 4046-4054. DOI:10.1021/acs.macromol.5b00423 |
| [12] |
G.H. Gao, J.W. Lee, M.K. Nguyen, et al., J. Control Release 155 (2011) 11-17. DOI:10.1016/j.jconrel.2010.09.012 |
| [13] |
N. Segovia, P. Dosta, A. Cascante, V. Ramos, S. Borrós, Acta Biomater. 10 (2014) 2147-2158. DOI:10.1016/j.actbio.2013.12.054 |
| [14] |
G.C. Yu, X.Y. Zhou, Z.B. Zhang, et al., J. Am. Chem. Soc. 134 (2012) 19489-19497. DOI:10.1021/ja3099905 |
| [15] |
M.V. Rekharsky, Y. Inoue, Chem. Rev. 98 (1998) 1875-1918. DOI:10.1021/cr970015o |
| [16] |
C. Li, G.F. Luo, H.Y. Wang, et al., J. Phys. Chem. C 115 (2011) 17651-17659. DOI:10.1021/jp203940s |
| [17] |
C. Xie, P. Zhang, Z.K. Zhang, et al., Nanoscale 7 (2015) 12572-12580. DOI:10.1039/C5NR02861B |
| [18] |
H. Han, D.E. Liu, H. Lu, W.X. Gu, H. Gao, RSC Adv. 4 (2014) 40882-40891. DOI:10.1039/C4RA07175A |
| [19] |
Y. Wang, D.D. Li, H.B. Wang, et al., Chem. Commun. 50 (2014) 9390-9392. DOI:10.1039/C4CC03978E |
| [20] |
D. Harries, D.C. Rau, V.A. Parsegian, J. Am. Chem. Soc. 127 (2005) 2184-2190. DOI:10.1021/ja045541t |
| [21] |
M.Y. Guo, M. Jiang, Soft Matter 5 (2009) 495-500. DOI:10.1039/B813556H |
| [22] |
J.M. Zhang, J.J. Li, Z. Shi, et al., Acta Biomater. 58 (2017) 349-364. DOI:10.1016/j.actbio.2017.04.029 |
| [23] |
R. Ji, J. Cheng, T. Yang, et al., Biomacromolecules 15 (2014) 3531-3539. DOI:10.1021/bm500711c |
| [24] |
D.E. Liu, H. Han, H.G. Lu, et al., RSC Adv. 4 (2014) 37130-37137. DOI:10.1039/C4RA04432K |
| [25] |
G.B. Zhu, X.H. Zhang, P.B. Gai, J.H. Chen, ChemPlusChem 77 (2012) 844-849. DOI:10.1002/cplu.201200144 |
| [26] |
X. Song, J.L. Zhu, Y.T. Wen, et al., J. Colloid Interface Sci. 490 (2017) 372-379. DOI:10.1016/j.jcis.2016.11.056 |
| [27] |
P. Zhang, X.P. Qian, Z.K. Zhang, et al., ACS Appl. Mater. Interfaces 9 (2017) 5768-5777. DOI:10.1021/acsami.6b14464 |
 2018, Vol. 29
2018, Vol. 29 
