b Shanghai Dermatology Hospital, Shanghai 200443, China;
c Department of Ophthalmology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200001, China
As the saying goes, the eyes are the windows of the mind. Ocular diseases not only directly affect human vision but also are closely bound up with quality of life. It is estimated that 285 million people from 39 countries suffer visual impairment between 2000 and 2010 [1]. Topical delivery of ocular therapeutics remains the preferable route for the treatment of ocular diseases concerning patient compliance. However, the specific anatomy and the normal physiology of the eye result in very poor ocular bioavailability of drugs [2]. The cornea, being composed of epithelium and endothelium with a centered stroma, forms the anatomic barrier for both lipophilic and hydrophilic drug molecules. Meanwhile, majority of topically applied drugs are lost due to the defense physiology of the eyes, e.g., eye blinking, tear film and turnover, and lacrimation. Less than 5% of the drug applied via eye drops eventually reaches the intraocular tissue [3]. Frequent instillation of eye drops are required to reach the therapeutic effect but may cause toxic side effects and damages to ocular tissues [4].
Nano drug delivery approaches have been explored extensively to improve the ocular bioavailability and proven to be a boon for ocular therapeutics [5, 6]. Among the diverse types of nanoparticles, nanoemulsions (NEs) and nanosuspensions (NSs) are two representative carriers. Nanoemulsions are composed of nano-size oil droplets which are stabilized by surfactant and co-surfactant and dispersed in aqueous phase [7, 8]. They can prolong drug residence time on precorneal surface and provide a higher penetration of drug into the eye [9-11]. Nanoemulsion based preparation has been marketed for ocular delivery of cyclosporine A (Restasis
Although prolonged retention and facilitated permeation are proposed to the enhanced ocular bioavailability of these nanocarriers, the virtual ocular fate of the intact carriers is still paradoxical. Aided by fluorescence labeling, some researchers support that nanoparticles can penetrate through ocular surface tissues to deliver encapsulated drugs to intraocular tissues [5, 21-26]. Others deny the entrance of the intact nanocarriers into the cornea, whereas the fluorescent markers passively redistributed from the vehicle to the lipophilic cellular compartments [27, 28]. The contradictory conclusions are derived from the undiscriminative probes. Signals from free probes may be mistaken for the nanocarriers. To accurately identify nanocarriers in vivo, it is crucial to take measures to discriminate nanocarriers from free probes [29].
Our group previously developed a kind of environmentresponsive fluorescent probes (Fig. S1 in Supporting information) with specific aggregation-caused quenching (ACQ) effects [30, 31]. The probes are hydrophobic and emit near infrared (NIR) fluorescence when molecularly dispersed. However, the fluorescence is instantly and completely quenched upon contacting with water due to formation of self-aggregated clusters through intermolecular π-π stacking. The probe molecules can be encapsulated in nanoemulsions [32-34] or embedded in the drug particles [35, 36] and emit near infrared (NIR) fluorescence. Once the probes are released from the carrier into aqueous environment, the fluorescence is quenched. Through the on–off signal switching, the probes can be used to monitor the integrity of the nanocarrier. Since water is ubiquitous in the body, the in vivo behavior of the intact carriers can be precisely tracked by the fluorescent signals of the ACQ probes [30-39].
In this study, we focused on the transocular behavior of intact nanoemulsions and nanosuspensions. Taking advantage of selfdiscriminative feature of the ACQ probes, we would like to provide solid evidence concerning the precorneal retention and transocular transportation of the intact carriers. Since Restasis
The mean particle size and PDI of NEs and NSs are presented in Table S1 (Supporting information). The mean size of two groups of NEs is 102 nm and 216 nm, respectively. The mean size of NSs is about 213 nm. For all nanocarriers, the PDI was less than 0.25, indicating narrow size distribution [41]. Besides, each group has similar fluorescent intensity. Fig. S2 (Supporting information) shows the morphology of NEs and NSs, which is spherical. The particle sizes observed are consistent with that measured by Zetasizer.
Fluorescent stability of the preparations in artificial tears is shown in Fig. 1. The fluorescence intensity of both two NEs is quite stable for eight hours, excluding interferences from fluorescent quenching due to water infiltration and leakage of probe molecules. However, a decrease of 13% in fluorescent intensity for NSs is observed. Comparing with NEs, NSs are apt to dissolve when suspended in water, leading to release of probe molecules and fluorescent quenching. However, previous studies proved that the fluorescent quenching is quantitatively related to the dissolution of NSs [36]. In this regard, fluorescent signals of ACQ probes can be used to track intact NEs and NSs.

|
Download:
|
| Fig. 1. Fluorescent stability of NEs and NSs incubated in artificial tear fluid | |
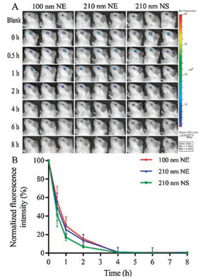
Live imaging shows the retention of nanocarriers in eyes (Fig. 2A). The fluorescence in the ocular region is decreasing as time goes by. About four hours post administration, fluorescence for all preparation almost disappears. However, the rates of fluorescent decreasing among three groups are different as shown in normalized fluorescence intensity percentage versus time curves (Fig. 2B). Generally, the retention of NEs is higher than NSs irrespective of particle size in the first 4 h of administration, although no significant difference was founded (P > 0.05). The reason may be ascribed to the dissolution of NSs. The dissolution of NSs in tears, being in agreement with the stability experiment, would release the encapsulated probes and cause their quenching. Conversely, the inner oil phases of NEs provide high affinity to the hydrophobic probe molecules and keeps the cargos away from the aqueous environment [42]. Although no significant difference was found between 100 nm NE and 210 nm ones (P > 0.05), the overall retention of smaller NEs is a little higher than the bigger ones as judged by the absolute value. That is because the smaller NEs have a larger specific surface area than the bigger ones [43, 44], leading to increased contact between the nanocarriers and the cornea and thus the prolonged retention [45]. However, CCA is a good adhesion agent, which may offset the differences of adhesion resulted from particle size, thus leading to no significant difference between two NEs groups.
|
Download:
|
| Fig. 2. Ocular retention of NEs and NSs. IVIS fluorescence image (A) and normalized fluorescence intensity percentage versus time curves (B) | |
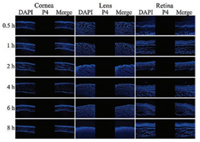
In order to observe the transaocular transportation of NEs and NSs, the animals were sacrificed at predetermined intervals and the eyeballs were removed. The cornea, lens and posterior segment (retina, choroid and sclera) were isolated. Following frozensection, the slices were observed under CLSM. Fig. 3 shows the tissues of the animals treated by quenched P4 solution. No fluorescence was observed in any of the tissues, indicating no fluorescence rekindling. The result excludes the interferences from fee probe molecules.
|
Download:
|
| Fig. 3. Fluorescence pictures of intraocular biodistribution of quenched P4 solution | |
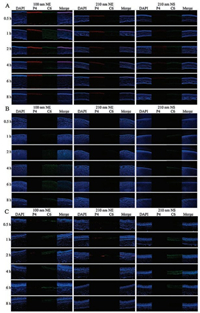
As shown in Fig. 4A, P4 signals (red) can be seen in the cornea of animals treated by all preparations, indicating the permeation of both two nanocarriers into the cornea. However, concerning the NE groups, the permeation is size-dependent. The 100 nm NE showed stronger fluorescent signals than the 210 nm one. The smaller NEs bear higher surface areas than the larger ones, facilitating the interaction with the ocular tissues. Besides, it is comparatively easier for smaller nanoparticles to pass through the biological barrier than the larger ones [46]. Consequently, decrease of particle size is advantageous for NEs to permeate into the cornea. Although intact NSs can also enter into the cornea, the efficiency is less than the NEs of similar size (Fig. 4A). For one reason, as shown in the results, precorneal retention of NS is inferior to NEs due to the dissolution, namely less intact NS are available on the surface of cornea. For another, the oil droplets of NEs might be more compatible to the lipid layer of the epithelium. However, irrespective of NEs and NSs, the P4 signal was mainly seen in the epithelium of the cornea. Only a faint signal can be found in either the stroma or the endothelium. The reason may be ascribed to the hydrophilic gel-like stroma, which occupies 90% of the corneal thickness and contains 80% water [47]. Thus it impedes the inward diffusion of the nano-vehicles into the cornea [48]. Conversely, the C6 signals (green), mimicking the cargos, can be seen throughout the three layers of the cornea. Particularly in the stroma and the endothelium, C6 signal is obviously stronger than P4. The results imply that the vehicles in the epithelium can serve as the depot and release the cargo molecules, which diffuse across the cornea.
|
Download:
|
| Fig. 4. Transocular transportation of NEs and NSs. CLSM imagines of the cornea (A), the lens (B), and the posterior segment (from top to down: retina, choroid and sclera) (C) | |
No P4 signals are observed in the lens of animals treated by all preparations, instead, C6 signals are seen (Fig. 4B). The results indicate that the vehicles cannot cross the cornea while the cargo molecules may diffuse through the tissue and reach the lens. Due to the tight junctions and desmosomes, corneal epithelia is almost impenetrable for any substances larger than 500 Da. Combined with the thick and hydrophilic stroma, it is difficult for the hydrophobic vehicles of NEs and NSs to penetrate into the inner side of cornea [17]. Even if a small amount of vehicles can reach the endothelium, they may not break through the inner barrier. Conversely, diffusion of cargo molecules (e.g., C6 with molecular weight of 350.4 Da) is comparatively easier than the vehicles. Even though, a lag time of 2 h to reach the maximum fluorescence intensity in the lens is found compared to the cornea, due to diffusion across the cornea (Figs. 4A and B).
Fig. 4C shows the CLSM photos of the posterior segment of animals treated by NEs and NSs. It is strange that strong C6 signals are found in these tissues with faint P4 signals. There are two routes for topically instilled formulation to reach the posterior parts of the eye, the anterior route and the conjunctival-scleral route. For anterior route, the formulation must penetrate across in sequence the cornea, the lens, the vitreous body and then reach the retina. Due to the long diffusion distance and the high resistance, the contribution of the anterior route is generally negligible [10]. The conjunctival-scleral route provides a shortcut to bypass the anterior segments. The formulation enters into the posterior part of the eye by crossing the conjunctiva. Then the formulation may take the trans-scleral transportation and reach the retina. At 0.5 h post administration, C6 signals can be seen in the posterior segment, while in the lens, the appearance of C6 signals is at 1 h. The time difference may support the conjunctival-scleral transportation. Thus, although further research is required, conjunctiva provides a potential route to deliver drugs to the back of eyes.
In conclusion, the intact NEs and NSs can be tracked by the aggregation-caused quenching probes. Following topical application, NEs show superior precorneal retention than NSs. Although both NEs and NSs cannot penetrate across the cornea, they can enter into the cornea and serve as depots. The smaller NEs show better permeation than the larger ones. Conversely, the released cargo molecules can diffuse across the cornea and enter into the anterior chamber. Moreover, the conjunctival-scleral route is potential to deliver drugs to the back of the eye.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 81872815, 81573363, 81690263, 21372063) and the Natural Science Foundation of Shanghai (No. 16ZR1403500).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.11.015.
| [1] |
Y. Weng, J. Liu, S. Jin, et al., Acta Pharm. Sin. B 7 (2017) 281-291. DOI:10.1016/j.apsb.2016.09.001 |
| [2] |
N.D. Sheybani, H. Yang, Chin. Chem. Lett. 28 (2017) 1817-1821. DOI:10.1016/j.cclet.2017.07.022 |
| [3] |
A. Tuomela, P. Liu, J. Puranen, et al., Int. J. Pharm. 467 (2014) 34-41. DOI:10.1016/j.ijpharm.2014.03.048 |
| [4] |
V.J. Rodríguez, V.L. Rodríguez, Int. J. Pharm. 531 (2017) 167-178. DOI:10.1016/j.ijpharm.2017.08.067 |
| [5] |
D.R. Janagam, L. Wu, T.L. Lowe, Adv. Drug Deliv. Rev. 122 (2017) 31-64. DOI:10.1016/j.addr.2017.04.001 |
| [6] |
M.L. Occhiutto, F.R. Freitas, R.C. Maranhao, V.P. Costa, Pharmaceutics 4 (2012) 252-275. DOI:10.3390/pharmaceutics4020252 |
| [7] |
Y. Lu, J. Qi, W. Wu, Curr. Drug Metab. 13 (2012) 396-417. DOI:10.2174/138920012800166544 |
| [8] |
W.H. Ji, Z.B. Xiao, G.Y. Liu, X. Zhang, Chin. Chem. Lett. 28 (2017) 1829-1834. DOI:10.1016/j.cclet.2017.06.024 |
| [9] |
S. Akhter, S. Talegaonkar, Z.I. Khan, et al., J. Biomed. Nanotechnol. 7 (2011) 144-145. DOI:10.1166/jbn.2011.1241 |
| [10] |
L. Ying, K. Tahara, H. Takeuchi, Int. J. Pharm. 453 (2013) 329-335. DOI:10.1016/j.ijpharm.2013.06.024 |
| [11] |
M. Gallarate, D. Chirio, R. Bussano, et al., Int. J. Pharm. 440 (2013) 126-134. DOI:10.1016/j.ijpharm.2012.10.015 |
| [12] |
M.J. Lawrence, G.D. Rees, Adv. Drug Deliv. Rev. 64 (2012) 175-193. DOI:10.1016/j.addr.2012.09.018 |
| [13] |
A. Radomska-Soukharev, J. Wojciechowska, Acta Pol. Pharm. 62 (2005) 465-471. |
| [14] |
Y. Lu, Y. Li, W. Wu, Acta Pharm. Sin. B 6 (2016) 106-113. DOI:10.1016/j.apsb.2015.11.005 |
| [15] |
Y. Lu, J. Qi, X. Dong, W. Zhao, W. Wu, Drug Discov. Today 22 (2017) 744-750. DOI:10.1016/j.drudis.2017.01.003 |
| [16] |
Y. Lu, Y. Chen, R.A. Gemeinhart, W. Wu, T. Li, Nanomedicine 10 (2015) 2537-2552. DOI:10.2217/nnm.15.73 |
| [17] |
R.D. Bachu, P. Chowdhury, Z.H.F. Al-Saedi, P.K. Karla, S.H.S. Boddu, Pharmaceutics 10 (2018) 28. DOI:10.3390/pharmaceutics10010028 |
| [18] |
A. Madni, M.A. Rahem, N. Tahir, et al., Int. J. Pharm. 530 (2017) 326-345. DOI:10.1016/j.ijpharm.2017.07.065 |
| [19] |
M.R. Gigliobianco, C. Casadidio, R. Censi, P.D. Martino, Pharmaceutics 10 (2018) 134. DOI:10.3390/pharmaceutics10030134 |
| [20] |
M. Kassem, A. Abdelrahman, M. Ghorab, M. Ahmed, R. Khalil, Int. J. Pharm. 340 (2007) 126-133. DOI:10.1016/j.ijpharm.2007.03.011 |
| [21] |
Y. Diebold, M. Calonge, Prog. Retinal Eye Res. 29 (2010) 596-609. DOI:10.1016/j.preteyeres.2010.08.002 |
| [22] |
X. Cai, S. Conley, M. Naash, Vision Res. 48 (2008) 319-324. DOI:10.1016/j.visres.2007.07.012 |
| [23] |
D. Thassu, G.J. Chader, Ocular Drug Delivery Systems:Barriers and Application of Nanoparticulate Systems, 1st ed., CRC Press, Florida (2012). |
| [24] |
H. Hillaireau, P. Couvreur, Cell. Mol. Life Sci. 66 (2009) 2873-2896. DOI:10.1007/s00018-009-0053-z |
| [25] |
E. Sakurai, H. Ozeki, N. Kunou, Y. Ogura, Ophthalmic Res. 33 (2001) 31-36. DOI:10.1159/000055638 |
| [26] |
K. Baba, Y. Tanaka, A. Kubota, et al., J. Control. Release 153 (2011) 278-287. DOI:10.1016/j.jconrel.2011.04.019 |
| [27] |
B.C. Tian, W.J. Zhang, H.M. Xu, et al., Colloids Surf. B -Biointerfaces 102 (2013) 251-256. DOI:10.1016/j.colsurfb.2012.08.021 |
| [28] |
S. Pretor, J. Bartels, T. Lorenz, et al., Mol. Pharm. 12 (2015) 34-45. DOI:10.1021/mp500401t |
| [29] |
X. Hu, X. Dong, Y. Lu, et al., Drug Discov. Today 22 (2017) 382-387. DOI:10.1016/j.drudis.2016.10.002 |
| [30] |
X. Hu, J. Zhang, Z. Yu, et al., Nanomedicine 11 (2015) 1939-1948. DOI:10.1016/j.nano.2015.06.013 |
| [31] |
X. Hu, W. Fan, Z. Yu, et al., Nanoscale 8 (2016) 7024-7035. DOI:10.1039/C5NR07474F |
| [32] |
R. Su, W. Fan, Q. Yu, et al., Oncotarget 8 (2017) 38214-38226. |
| [33] |
F. Xia, W. Fan, S. Jiang, et al., ACS Appl. Mater. Interfaces 9 (2017) 21660-21672. DOI:10.1021/acsami.7b04916 |
| [34] |
E. Ahmad, Y. Feng, J. Qi, et al., Nanoscale 9 (2017) 1174-1183. DOI:10.1039/C6NR07581A |
| [35] |
C. Shen, Y. Yang, B. Shen, et al., Nanoscale 10 (2017) 436-450. |
| [36] |
Y. Xie, B. Shi, F. Xia, et al., J. Control. Release 270 (2018) 65-75. DOI:10.1016/j.jconrel.2017.11.046 |
| [37] |
Y. Xie, X. Hu, H. He, et al., J. Mater. Chem. B -Mater. Biol. Med. 4 (2016) 2864-2873. DOI:10.1039/C5TB02706C |
| [38] |
B. Wan, Y. Dai, D. Liu, et al., J. Biomed. Nanotechnol. 13 (2017) 960-972. DOI:10.1166/jbn.2017.2404 |
| [39] |
H. He, J. Zhang, Y. Xie, et al., Mol. Pharm. 13 (2016) 4013-4019. DOI:10.1021/acs.molpharmaceut.6b00705 |
| [40] |
Z. Rahman, X. Xu, U. Katragadda, et al., Mol. Pharm. 11 (2014) 787-799. DOI:10.1021/mp400484g |
| [41] |
M.J. Newton Rimple, Recent Pat. Inflamm. Allergy Drug Discov. 12 (2018) 169-183. DOI:10.2174/1872213X12666180730115225 |
| [42] |
C. Luschmann, W. Herrmann, O. Strauss, K. Luschmann, A. Goepferich, Int. J. Pharm. 455 (2013) 331-337. DOI:10.1016/j.ijpharm.2013.07.002 |
| [43] |
H.D. Williams, N.L. Trevaskis, S.A. Charman, et al., Pharmacol. Rev. 65 (2013) 315-499. DOI:10.1124/pr.112.005660 |
| [44] |
R.H. Müller, S. Gohla, C.M. Keck, Eur. J. Pharm. Biopharm. 78 (2011) 1-9. DOI:10.1016/j.ejpb.2011.01.007 |
| [45] |
C.G. Park, Y.K. Kim, M.J. Kim, et al., J. Control. Release 220 (2015) 180-188. DOI:10.1016/j.jconrel.2015.10.027 |
| [46] |
U.B. Kompella, A.C. Amrite, R. Pacha Ravi, S.A. Durazo, Prog. Retinal Eye Res. 36 (2013) 172-198. DOI:10.1016/j.preteyeres.2013.04.001 |
| [47] |
M. Rawas-Qalaji, C.A. Williams, Curr. Eye Res. 37 (2012) 345-356. DOI:10.3109/02713683.2011.652286 |
| [48] |
Y. Chen, Y. Lu, Y. Zhong, et al., J. Drug Target. 20 (2012) 856-863. DOI:10.3109/1061186X.2012.723214 |
 2018, Vol. 29
2018, Vol. 29 
