b Department of Geriatrics, The Second Affiliated Hospital, Key Laboratory for Aging & Disease, Nanjing Medical University, Nanjing 210011, China;
c College of Life Science, Yangtze University, Jingzhou 434025, China;
d Department of Technical Services, Jiangsu Provincial Center for Disease Prevention and Control, Nanjing 210009, China
Staphylococcus aureus is a round-shaped, Gram-positive bacterium, which is a normal microbiota present in nose, upper respiratory tract and skin of approximately one third population of healthy human. S. aureus is flexible and adaptable to severe environment such as hypersaline, heat and dryness, therefore it is a common factor for a large number of human infection diseases including soft-tissue infection, pneumonia, meningitis, toxic shock syndrome or bacteremia. S. aureus is notorious for developing extensive resistance to antibiotics in a variety of ways. The first methicillin-resistant S. aureus (MRSA) was described in England (1961) and spread rapidly because of the antibiotic selection and the horizontal transfer of resistance genes [1]. In response, vancomycin is widely used to treat the clinical MRSA strains, but the prevalence of vancomycin-resistant S. aureus (VRSA) strains has become increasingly all over the world, making that the alternate therapeutic strategy is urgently expected [2, 3].
Silver (Ag) as a natural antiseptic has been used to treat the bacterial infection for hundreds of years. Recently, with the development of nano-technology, silver nanoparticles (AgNPs) have achieved considerable attention as novel antimicrobial agents due to their high surface area to volume ratio and excellent physical and chemical properties [4-10]. Because of the potent broad-spectrum antimicrobial property with low toxicity to human beings, AgNPs have been widely used in different biomedical products including bandages, catheters, textiles, and even drug delivery system [11-18]. However, the widespread and indiscriminate use of nanosilver brings not only serious environmental pollution, but also the prevalence and spread of nanosilverresistance microorganisms [19, 20]. Spermine is a low-molecular weight natural compound with positively charged amine groups existing at millimolar levels in all living cells, which plays important roles in the regulation of physical and chemical properties of membranes, nucleic acid structures, and enzyme activities by interacting with negatively charged macromolecules in cytoplasm [21, 22]. According to the conclusion reported in the literature, exogenous spermine at low concentrations could suppress the growth of S. aureus potently, several studies indicate that the spermine increases the antibiotics activity on the growth of Escherichia coli, Salmonella enterica, Pseudomonas aeruginosa and Staphylococcus aureus, but the interaction mechanism between spermine and AgNPs is still poorly understood [23-27].
To assess the antimicrobial effect of AgNPs in combination with spermine, MRSA strains were identified and collected according to CLSI standard, and routinely grown on Luria-Bertani (LB) agar plates or broth at 37 ℃ under normal aeration [28]. Silver nitrate, spermine tetrahydrochloride, and other chemicals used in this study were purchased from Sigma-Aldrich. Colloidal AgNPs solution was prepared as described previously with some modifications [29]. A total of 90 mL double-distilled water was added in a round bottom flask to heated boiling for 5 min, then 2.0 mL of 1% (w/v) citrate solution and 0.56 mL of 1% (w/v) AgNO3 solution were introduced to the mixture in turn, followed by the quick addition of 0.66 mL of 0.1% (w/v) freshly prepared NaBH4 solution. The reaction solution was kept at boiling under vigorous stirring for 1 h and cooled to room temperature, and distilled water was added to bring the volume of the dispersion to 100 mL. UV–vis spectra of these samples were monitored by Shimadzu UV-3600 spectrophotometer in the range of 300–800 nm, transmission electron microscopy (TEM) images were taken with a JEM-2100EX (JEOL) transmission electron microscope operated at 200 kV. Minimum inhibitory concentration (MIC) of AgNPs and spermine was then measured by broth microdilution method [28], fresh overnight cultures of each isolate were diluted in Mueller-Hinton (MH) (Oxoid) broth (pH 7.0) to get approximately 1 ×106 viable cells per milliliter and incubated overnight (16–18 h) at 37 ℃ without shaking, MIC values were determined as the lowest concentration that inhibited cellular growth completely. Then a checkerboard assay was performed by determining the FICI described by Timurkaynak et al. [30], fresh overnight cultures of each isolate were inoculated to each AgNPs (512 μg/mL)/spermine (0.25–16 mmol/L) combination in the array and incubated at 37 ℃ for 18 h, and FICI was calculated as follows: FICI = MIC (AgNPs + spermine)/MIC (AgNPs) + MIC (AgNPs + spermine)/MIC (spermine). The synergy was defined as FIC index ≤0.5, partial synergy was defined as FIC index >0.5 but ≤0.75, additive effect was defined as FIC index >0.76 but ≤1, indifference was defined as FIC index >1 but ≤4, and antagonism was defined as FIC index >4 [30]. The growth curve and time-killing assay of MRSA strain were also measurement, overnight bacterial cultures were diluted into fresh LB medium to OD600 of 0.1, aliquots of 100 μL of each strain were distributed in triplicate into the wells of a flat bottom 96-well microtiter plate and then incubated in reader at 37 ℃ with intermittent shaking, growth curve was monitored by measuring the absorbance at 600 nm every 2 h for 16 h. Time-killing assay was performed for determine synergist effect between AgNPs and spermine, MRSA cells (~1 ×105 CFU) was inoculated into 1 mL of the LB broth at 37 ℃ in the presence or absence of the AgNPs or spermine. After incubation, bacteria were harvested at the indicated time points (0, 4, 8, and 12 h) and 100-μL aliquots were taken from each sample to determine the number of colonyforming units (CFUs). All samples were plated in triplicate and values were averaged from three independent experiments.
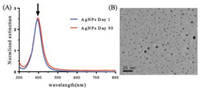
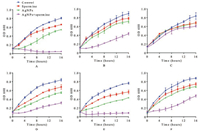
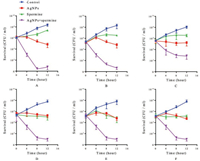
In this study, AgNPs were synthesized involving NaBH4 reduction of AgNO3 in the presence of citrate solution at 100 ℃. Along with the reaction process, yellow color mixture appeared progressively, indicating the formation of silver nanoparticles. The excitation spectra of reaction mixture showed a single strong peak at 395 nm (Fig. 1A). TEM image showed that AgNPs were in a narrow size distribution of about 5 nm (Fig. 1B). MIC values showed that both AgNPs and spermine exhibited inhibitory activities against MRSA isolates (Table 1). The MICs of AgNPs and spermine in all tested strains were 256 μg/mL and 2 mmol/L, respectively. In checkerboard dilution test (Table 1), spermine markedly enhanced the antibacterial capacity of silver nanoparticles. All of the tested strains exhibited 2 to 16-fold reduction in MIC values, the combined activity of spermine and AgNPs against clinical MRSA strains resulted in FICIs ranging from 0.375 to 1. It indicated that spermine can be served as excellent adjuvant with AgNPs to against the growth of S. aureus. Growth curves of MRSA strains were determined in the presence of subinhibitory concentration (1/4 MIC) of AgNPs and spermine, the growth of most MRSA strains was unaffected by 64 μg/mL AgNPs or 0.5 mmol/L spermine alone, but obviously inhibited by the combination in same concentration (Fig. 2). Time-killing assay (Fig. 3) showed MRSA strains in MH broth was not killed by subinhibitory concentration (1/2 MIC) of AgNPs (128 μg/mL) or spermine (1 mmol/L) alone, but the combination of AgNPs and spermine inhibited the growth of MRSA strains obviously, nearly all initial inoculums were killed during first 8 h. However, it is noteworthy that not all MRSA strains were inhibited by this recipe effectively, the survival of clinical isolate MRSA-29-217 was only gets a small influence in time-killing assay.
|
Download:
|
| Fig. 1. (A) UV–vis absorption spectra of the AgNPs solution. The arrow indicates the maximum absorbance at 395 nm. (B) TEM image of the AgNPs with the scale bar of 20 nm | |
|
Download:
|
| Fig. 2. The growth rate of MRSA strains in the presence or absence of subinhibitory AgNPs (64 μg/mL) and spermine (0.5 mmol/L). (A) isolate ATCC BAA-1026; (B) isolate MRSA-18-122; (C) isolate MRSA-29-217; (D) isolate MRSA-29-238; (E) isolate MRSA-29-256; (F) isolate MRSA-29-227 | |
|
Download:
|
| Fig. 3. Time-killing curve of MRSA isolates in the presence of spermine and/or AgNPs. Time-killing assay was performed in the MH broth with control (blue circle), AgNPs alone (128 μg/mL, red square), spermine alone (1 mmol/L, triangle), and both (purple inverted triangle). (A) isolate ATCC BAA-1026; (B) isolate MRSA-18-122; (C) isolate MRSA-29-217; (D) isolate MRSA-29-238; (E) isolate MRSA-29-256; (F) isolate MRSA-29-227 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article) | |
|
|
Table 1 Antimicrobial activity of AgNPs and spermine against MRSA strains.a, b |
The struggle between human and communicable diseases has never stopped since time immemorial. During that time, silver was once the optimal selection for medical treatment to the open wounds and burns by the means of its antiseptic property [31]. Up to the early 1940s, various antibiotics were invented and widely used in the clinical treatment, it is being called one of the greatest inventions during the past one hundred years, millions of people's lives have been saved by this "elixir" since the onset of their used in therapy. However, with the indiscriminate use of it, increasing number of bacteria evolved resistance antibiotics by numerous mechanisms have been spotted, causing great cost of treatment and increasing the sufferings of patients. Therefore, the alternate and effective therapeutic strategies to treat the antibiotic-resistant pathogens are imminent [32, 33].
In recent years, with the rapid development of nanotechnology, nanomaterials have attracted wide attention due to their unique antibacterial properties [34-38]. The metallic nanoparticles including copper, titanium, magnesium, zinc and gold were well known for their antibacterial properties, but silver nanoparticles have been proven the most potent antimicrobial agent among them [6, 8, 39, 40]. AgNPs are considered to be bactericidal by diversified weapons including Ag+-induced membrane damage, Ag+-related ROS production, DNA mutation and ATP yields decreased caused by Ag+ ions, and site-specific enzyme inhibition. Along with the widely used in various fields of AgNPs, the problem of bacterial silver ions resistance and environmental pollution caused by a large amount industrial production and indiscriminate application of silver products needs worth consideration. Therefore, the scientific and rational use of silver nanoparticles with some adjuvants to improve the antibacterial efficiency of AgNPs is of great value.
The antibacterial effect of AgNPs has been proven to be improved by decreasing their particle size [41]. In this study, silver nanoparticles with diameter ~5 nm were synthesized, and the MICs of AgNP to MRSA strain was 256 μg/mL. To enhance the antibacterial capacity of AgNPs, spermine was added as adjuvant, the results of checker board test and time-killing curves showed that spermine possessed not only antimicrobial activities alone but also additive effects with AgNPs in MRSA strain treatment, only MRSA-29-217 cannot be effectively suppressed by this recipe, the underlying mechanism for this phenomenon is still need to be further investigated.
Spermine was first described at 1677 by Antonie van Leeuwenhoek in human semen, which belongs to biogenic polyamines and involved in cellular metabolism in most eukaryotic and prokaryotic cells. The role of spermine in bacterial antimicrobial resistance is still under discussion. On one hand, it has been widely demonstrated that polyamines can act as endogenous modulators of outer membrane permeability of bacteria to make resistance to cationic peptide, aminoglycoside or quinolone antibiotics [25, 42]. On the other hand, spermine enhances the susceptibility to β-lactams in several bacteria including Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, Salmonella enterica and S. aureus [24-26, 43]. The molecular target of spermine toxicity in S. aureus remains largely unknow, but some MRSA strains, such as the dominant community-associated strain USA300, are resistant to spermine due to acquisition of the speG gene found on the mobile ACME element [44]. In A. baumannii, spermine was considered to decrease the levels of intracellular glutathione, causing the spermine-mediated β-lactam susceptibility, but it is still not clear whether this mechanism plays the same role in S. aureus.
Accordingly, this study demonstrates that spermine can effectively reinforce the antibacterial activity of AgNPs, suggesting that spermine has bright application prospects as an adjuvant to AgNPs for curing various infectious diseases as well as substantially reducing the use of silver antibacterial products.
AcknowledgementsThis research was supported by the Fundamental Research Funds for the Jiangsu Province Medical Talent (No. ZDRCA2016065), Science and Technology Support Project of Jiangsu Province (No. BE2017763), Central Universities (No. 3332018178) and Jiangsu Planned Projects for Postdoctoral Research Funds (No. 2018K240C).
| [1] |
A.S. Lee, H. de Lencastre, J. Garau, et al., Nat. Rev. 4 (2018) 18033. |
| [2] |
I.M. Gould, Int. J. Antimicrob. Agents 42 (2013) 17-21. |
| [3] |
T.Y. Zhang, C. Li, Y.S. Tian, et al., Chin. Chem. Lett. 28 (2017) 1737-1742. DOI:10.1016/j.cclet.2017.05.022 |
| [4] |
A.F. Martins, J.P. Monteiro, E.G. Bonafé, et al., Chin. Chem. Lett. 26 (2015) 1129-1132. DOI:10.1016/j.cclet.2015.04.032 |
| [5] |
S.M. Shaban, I. Aiad, M.M. El-Sukkary, et al., Chin. Chem. Lett. 28 (2017) 264-273. DOI:10.1016/j.cclet.2016.09.010 |
| [6] |
S. Li, S. Dong, W. Xu, et al., Adv. Sci. (Weinh.) 5 (2018) 1700527. |
| [7] |
S. Ullah Khan, T.A. Saleh, A. Wahab, et al., Int. J. Nanomed. 13 (2018) 733-762. DOI:10.2147/IJN |
| [8] |
R.Y. Pelgrift, A.J. Friedman, Adv. Drug Deliv. Rev. 65 (2013) 1803-1815. DOI:10.1016/j.addr.2013.07.011 |
| [9] |
M.A. Syed, S. Babar, A.S. Bhatti, H. Bokhari, J. Biomed. Nanotechnol. 5 (2009) 209-214. DOI:10.1166/jbn.2009.1020 |
| [10] |
Y. Li, Y. Chang, X. Lian, et al., J. Biomed. Nanotechnol. 14 (2018) 1515-1542. DOI:10.1166/jbn.2018.2614 |
| [11] |
X. Wang, L. Zhang, J. Wang, et al., J. Biomed. Nanotechnol. 14 (2018) 1448-1457. DOI:10.1166/jbn.2018.2601 |
| [12] |
Y. Nishimura, J. Ishii, C. Ogino, A. Kondo, J. Biomed. Nanotechnol. 10 (2014) 2063-2085. DOI:10.1166/jbn.2014.1951 |
| [13] |
X. Chen, H.J. Schluesener, Toxicol. Lett. 176 (2008) 1-12. DOI:10.1016/j.toxlet.2007.10.004 |
| [14] |
L. Wei, J. Lu, H. Xu, et al., Drug Discov. Today 20 (2015) 595-601. DOI:10.1016/j.drudis.2014.11.014 |
| [15] |
L. Rizzello, P.P. Pompa, Chem. Soc. Rev. 43 (2014) 1501-1518. DOI:10.1039/C3CS60218D |
| [16] |
A. Schroeter, T. Engelbrecht, R.H. Neubert, A.S. Goebel, J. Biomed. Nanotechnol. 6 (2010) 511. DOI:10.1166/jbn.2010.1149 |
| [17] |
B. Baroli, J. Biomed. Nanotechnol. 6 (2010) 485-496. DOI:10.1166/jbn.2010.1147 |
| [18] |
L. Xu, R. Bai, X. Cheng, et al., J. Biomed. Nanotechnol. 14 (2018) 564-574. DOI:10.1166/jbn.2018.2534 |
| [19] |
H. Haase, A. Fahmi, B. Mahltig, J. Biomed. Nanotechnol. 10 (2014) 1146-1156. DOI:10.1166/jbn.2014.1784 |
| [20] |
C. Gunawan, C.P. Marquis, R. Amal, et al., ACS Nano 11 (2017) 3438-3445. DOI:10.1021/acsnano.7b01166 |
| [21] |
A. Gugliucci, Clin. Chim. Acta 344 (2004) 23-35. DOI:10.1016/j.cccn.2004.02.022 |
| [22] |
E. Agostinelli, G. Arancia, L.D. Vedova, et al., Amino Acids 27 (2004) 347-358. DOI:10.1007/s00726-004-0114-4 |
| [23] |
M. Zhang, H. Wang, K.J. Tracey, Crit. Care Med. 28 (2000) 60-66. |
| [24] |
D.H. Kwon, C.D. Lu, Antimicrob. Agents Chemother. 51 (2007) 2070-2077. DOI:10.1128/AAC.01472-06 |
| [25] |
D.H. Kwon, C.D. Lu, Antimicrob. Agents Chemother. 50 (2006) 1615-1622. DOI:10.1128/AAC.50.5.1615-1622.2006 |
| [26] |
D.H. Kwon, C.D. Lu, Antimicrob. Agents Chemother. 50 (2006) 1623-1627. DOI:10.1128/AAC.50.5.1623-1627.2006 |
| [27] |
D.H. Kwon, S. Hekmaty, G. Seecoomar, Antimicrob. Agents Chemother. 57 (2013) 5457-5461. DOI:10.1128/AAC.00692-13 |
| [28] |
C.L.S. Institute, M100-S20 30 (2010). |
| [29] |
Y. Wan, Z. Guo, X. Jiang, et al., J. Colloid Interface Sci. 394 (2013) 263-268. DOI:10.1016/j.jcis.2012.12.037 |
| [30] |
F. Timurkaynak, F. Can, O.K. Azap, et al., Int. J. Antimicrob. Agents 27 (2006) 224-228. DOI:10.1016/j.ijantimicag.2005.10.012 |
| [31] |
M.K. Rai, S.D. Deshmukh, A.P. Ingle, A.K. Gade, J. Appl. Microbiol. 112 (2012) 841-852. DOI:10.1111/jam.2012.112.issue-5 |
| [32] |
M.T. Gabr, N.S. El-Gohary, E.R. El-Bendary, et al., Chin. Chem. Lett. 26 (2015) 1522-1528. DOI:10.1016/j.cclet.2015.09.004 |
| [33] |
M. Arshad, A.R. Bhat, K.K. Hoi, et al., Chin. Chem. Lett. 28 (2017) 1559-1565. DOI:10.1016/j.cclet.2016.12.037 |
| [34] |
W.A. Velema, W. Szymanski, B.L. Feringa, J. Am. Chem. Soc. 136 (2014) 2178-2191. DOI:10.1021/ja413063e |
| [35] |
W.A. Velema, J.P. van der Berg, M.J. Hansen, et al., Nat. Chem. 5 (2013) 924-928. DOI:10.1038/nchem.1750 |
| [36] |
K. Stranius, K. Borjesson, Sci. Rep. 7 (2017) 41145. DOI:10.1038/srep41145 |
| [37] |
H. Qian, Y.Y. Wang, D.S. Guo, I. Aprahamian, J. Am. Chem. Soc. 139 (2017) 1037-1040. DOI:10.1021/jacs.6b10982 |
| [38] |
Y. Liu, H.C.V.D. Mei, B. Zhao, et al., Adv. Funct. Mater. 27 (2017) 1701974. DOI:10.1002/adfm.v27.44 |
| [39] |
J. Xia, W. Wang, X. Hai, et al., Chin. Chem. Lett. (2018). DOI:10.1016/j.cclet.2018.07.008 |
| [40] |
J. Lin, J. Ding, Y. Dai, et al., Mater. Sci. Eng. C:Mater. Biol. Appl. 81 (2017) 321-326. DOI:10.1016/j.msec.2017.08.009 |
| [41] |
M.E. Samberg, P.E. Orndorff, N.A. Monteiro-Riviere, Nanotoxicology 5 (2011) 244-253. DOI:10.3109/17435390.2010.525669 |
| [42] |
A.L. Dela Vega, A.H. Delcour, J. Bacteriol. 178 (1996) 3715-3721. DOI:10.1128/jb.178.13.3715-3721.1996 |
| [43] |
L. Malone, D.H. Kwon, Int. J. Antimicrob. Agents 41 (2013) 70-74. DOI:10.1016/j.ijantimicag.2012.08.009 |
| [44] |
G.S. Joshi, J.S. Spontak, D.G. Klapper, A.R. Richardson, Mol. Microbiol. 82 (2011) 9-20. DOI:10.1111/mmi.2011.82.issue-1 |
 2018, Vol. 29
2018, Vol. 29 

