b Department of Gynecology and Obstetrics, West China Second University Hospital, Sichuan University, Chengdu 610041, China;
c Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu 610041, China;
d National-Regional Key Technology Engineering Laboratory for Medical Ultrasound, Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, School of Biomedical Engineering, Shenzhen University, Shenzhen 518060, China
Camptothecin (CPT) is a kind of strong anti-cancer natural compound that extracted from our country's unique Davidiaceae plant happy tree, and it has a strong tumor killing ability for a variety of tumor cells with its special anti-cancer mechanism including the breast cancer [1-4]. More than forty percent of active anti-disease substances being screened by combinatorial screening programs possess hydrophobic nature. Such a hydrophobic character makes them difficult to be directly developed as drug products for clinical translations because the low water solubility will strongly prevent them from being administrated through intravenous route [5-8]. CPT has low bioavailability and encapsulation efficiency, limiting its clinical use [9, 10]. Chemical modification of drug molecules is the typical solution to make them more water soluble. But it will result in the loss of anti-disease activity and significant alterations in the toxicological features of the drug molecules [11-14]. Thus, alternative strategies to traditional chemical modifications for improved drug water solubility are highly recommended [15]. So, we introduce a nanocarriers, hollow mesoporous silica nanoparticles (HMSNs) with unique hollow cavity and mesoporous structure, that has been explored as many fields such as separations, bio-imaging and drug delivery, especially an effective drug delivery system for a variety of therapeutic agents to fight against various kinds of diseases [16-20]. The hollow mesoporous silica shell with controllable thickness, hydrophilic inner/outer surface, tunable pore size, large hollow cavity and surface, brings remarkable ability to drug loading capacity [21-28]. In addition, hollow mesoporous silica nanoparticles have high stability, excellent biocompatibility, and good degradability [29-37].
CPT was one of the effective drugs for the breast cancer. Breast cancer has been a deadly disease that continues to disrupt the lives of millions of women and their families worldwide [38, 39], and it affects one in eight women in the United States. Despite of much advances in varieties of treatment modalities, local relapse remains a serious clinical problem, especially the patients who underwent breast-conserving therapy (BCT) faced with great postoperative recurrence problems [40-42]. Meanwhile we successfully synthesized hollow mesoporous silica nanoparticles as efficient encapsulation and intracellular delivery of hydrophobic anticancer drugs.
Because local therapy is essential to prevent the local relapse, even the metastasis and hydrogels are the excellent choice for local therapy because of controlled drug release locally, reducing the side effects of the drugs [43-45]. They exhibit distinguished potential as drug carrier for local delivery. At the same time, their intelligent three-dimensional structural changes in response to environmental stimuli such as temperature, H2O2, pH, light, and electrical fields generates drug control release [46-51]. According to the previous reports [52], we have synthesized a series of thermosensitive hydrogels that formed a flowing state at room temperature but changed to a gel state at physiological temperature. Therefore, the HMSNs loading CPT and PDLLA-PEG-PDLLA (PLEL) hydrogel were combined for the effective local therapy to prevent the local breast cancer recurrence.
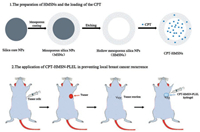
In this study the drug CPT, that had to modify the structure to make them more water soluble for injection in most reports, was loaded into the HMSNs with the high loading capacity. Then for local therapy to prevent the recurrence of breast cancer, the CPT@HMSNs were loaded into the PLEL thermosensitive hydrogels. After the tumor was resected, the CPT-HMSNs-PLEL system was injected into the tumor site and the CPT could be released continuously locally (Scheme 1).
|
Download:
|
| Scheme 1. Schematic illustrating the preparation of CPT-HMSN and the application of CPT-HMSN-PLEL in preventing local breast cancer recurrence | |
We prepared the HMSNs successfully. We synthesized the nonporous spherical silica core (Fig. 1a), and from the TEM images we can see that the average diameter of the core is about 80 nm. The second step was to create the mesoporous shell around these spheres cores, and the average diameter of one shell is about 20 nm (Fig. 1b). Then we dissolved the core to get hollow mesoporous silica nanoparticles. From the Figs. 1c and d, we could see the homogeneous cavity and mesoporous about the silica nanoparticles.

|
Download:
|
| Fig. 1. TEM images of (a) silica core NPs, (b) Mesoporous silica NPs, (c, d) Hollow mesoporous silica NPs | |
Then the CPT was loaded into the HMSNs and the drug loading capacity of CPT was evaluated by thermal weight loss (TG) and the high performance liquid chromatography (HPLC). The CPT will lose weight following the temperature increment but silica will not. In Fig. S1 (Supporting information), we can get a very high drug loading capacity of CPT that is about 66.04%, which has greatly decreases the expenditure of silica nanoparticles. At the same time, to exclude the influence of other impurities we measured the drug loading capacity of CPT though HPLC and value of number was almost the same. These results demonstrated that we have successfully get CPT-loaded HMSNs and we get a high enough drug loading capacity of CPT that is about 66.04%, which has greatly enhanced the drug loading capacity of hydrophobic CPT, decreased the expenditure of biological materials, reduced the biological toxicity.
The safety of the HMSN was significant. To measure interaction between HMSNs and the blood cells, we focused on the hemolytic effect of the materials. The hemolytic effect was evaluated by the method of semi-quantitative detection of the concentration of hemoglobin after co-culture with blood cells and visual observation and measurement of ruptured cells. In Fig. S2A (Supporting information), it shows that the solution of the samples with different concentrations of HMSNs solution incubated with blood cells for 3 h. In the negative control (saline) solution, the supernatant was colorless and clear, the red blood cells all sink to the bottom of the tube and no hemolysis occurred. The positive control (ultrapure water) solution showed uniform redness and obvious hemolysis occurred. The supernatant of the HMSNs solution with the concentration of 0.125–2 mg/mL was colorless and transparent, so no obvious hemolysis occurred. Taking into account of the centrifugation, transfer and other operations and a small amount of cell rupture brought about by the background, we can think that HMSNs materials will not cause hemolytic reaction.
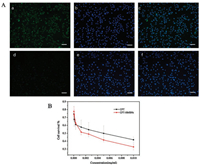
|
Download:
|
| Fig. 2. (A) (a–c) In vitro cellular uptake of Cou-HMSNs, (d–f) In vitro cellular uptake of blank PBS (scale bar = 200 μm). (B) Cell viabilities of 4T1 at different dosages of CPT and CPT-HMSNs | |
3T3 cells were used to evaluate the safety of the HMSNs. It can be seen from Fig. S2B (Supporting information) that the measured concentration of the HMSNs showed low cytotoxicity to 3T3 cells, and the cells were still able to proliferate well and there was no significant difference compared with the control group.
In Fig. 2A, it shows the cellular uptake studies about the HMSNs loading the Cou, a hydrophobic fluorescence probe, coumarin (Cou), was selected as a model drug of CPT. The left was coumarin fluorescence (green), the middle of the silica coumarin HMSNs/Cou DAPI staining to locate the nucleus (blue), the right side of the two superimposed. It is clear that the fluorescent HMSNs are enriched in the nucleus and there is almost no in the cytoplasm after 4 h coculture. It means that HMSNs can be pumped into the nucleus and will help CPT play anti-tumor effect that could the rapid cleavage of tumor cells in the mitotic stage is firmly fixed, thus microtubules are no longer separated. It can block cells in the cell cycle G2 and M phase, so that cancer cell replication blocked and died.
Then the cytotoxicity of HMSNs loading the CPT was investigated by MTT method on 4T1 cells. By comparing the free drug group, it could be seen that the viability of the cells did not reduce in the HMSNs loading CPT that even be better than the free (Fig. 2B). So the HMSNs can be used as a good carrier of CPT for follow-up study.
For local therapy, the CPT-HMSN was loaded into the PLEL thermosensitive hydrogel. The release behavior and the rheological characteristic about the CPT-HMSNs-PLEL hydrogel were investigated.
The release behavior of CPT drugs can be determined by HPLC.The drug release was observed by the experimental results in Fig. S3 in Supporting information. Free CPT, CPT-HMSN, hydrogel loading CPTHMSN, hydrogel loading CPT were placed in PBS buffer at pH 7.4, respectively. The drug release was observed by the experimental results.The drug has released completely on the fourth day in the free group. And the CPT-HMSNs was 46.9% and CPT-PLEL was 59.74%, however the group of CPT-HMSNs-PLEL was just about 30%. Both CPT-HMSNs and CPT-PLEL achieved the cumulative release of 100% after 15 days, thus CPT-HMSN-PLEL just reached about 60% which demonstrated the excellent sustained release effect.
The dynamic rheological measurements were carried out with PLEL hydrogel and HMSNs-PLEL hydrogel. According to our previous reports, the PLEL demonstrated the thermosensitivity. In Fig. S4 (Supporting information), there was no obvious difference for the G0 between PLEL hydrogel and HMSNs-PLEL hydrogel. The G0 of them both increased observably at about 30 ℃ and with the further increase of temperature, it decreased obviously at about 38 ℃. It means that HMSNs-PLEL hydrogel had obvious thermosensitive.
The most important, we assessed whether CPT-HMSNs-PLEL hydrogels prevented local recurrence of breast cancer in vivo after primary tumor resection. We randomized mice into eight groups after resection of the primary tumor. All mice were almost the same (tumor volume and weight) before resection and characteristics of primary tumors among the eight experimental groups. During the 30 days for observation of, all the mice in control group steadily developed tumors on the second day after operation. Thus the recurrences of the HMSNs-PLEL group, CPT-PLEL (0.5 mg/kg) group and CPT-PLEL (1 mg/kg) group were also 100%, but recurrences were postponed. Fortunately, the recurrence rate of CPT-HMSNs-PLEL hydrogels was much lower than other groups. Six of seven mice with CPT-HMSNs-PLEL (0.5 mg/kg, 86%), five of eight mice with CPT-HSNs-PLEL (1 mg/kg, 62%), 4 of 9 mice with CPT-HMSNs-PLEL (2 mg/kg, 44%) developed tumors. These results demonstrate that recurrence rate in vivo was much more suppressed by the CPT-HMSNs-PLEL hydrogel compared with other groups (Fig. 3A).

|
Download:
|
| Fig. 3. (A) In vivo breast cancer locoregional recurrence. (B) Body weight variation after treatment | |
The application of CPT was limited due to the greatly cancer cell cytotoxicity, and it also could bring some serious side effects. All mice appeared mildly losing weight on account of the surgery. Over time, the mice gradually recovered from the operative period and showed a stable trend of weight change (Fig. 3B). Therefore the system reduced the systemic toxicity.
By histopathology, there was no obvious toxicological pathology (Fig. S5 in Supporting information). This result shows that the HMSNs-PLEL hydrogels reduced the toxicity of CPT, the HMSNsPLEL system is safe.
In summary, we had successfully synthesized the HMSNs that could load CPT with the high loading capacity. The CPT-HMSNs was loaded into the PLEL thermosensitive hydrogel for local therapy in preventing breast cancer recurrence after primary tumor resection in a mouse model. The CPT-HMSN-PLEL system could release drugs in a consistent and sustained fashion and decreased systemic toxicity of CPT. It could prevent the local relapse, even the metastasis for the breast cancer.
AcknowledgementsThis work was supported by the National Natural Science Foundation of China (Nos. 31700869, 31700868, 31771096), the National Science Fund for Distinguished Young Scholars (No. NSFC31525009), the Fundamental Research Funds for the Central Universities/the Postdoctoral Research Foundation of Sichuan University (Nos. 2017SCU12032, 2017SCU12040), and Sichuan Innovative Research Team Program for Young Scientists (No. 2016TD0004).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.10.004.
| [1] |
M. Berrada, A. Serreqi, F. Dabbarh, et al., Biomaterials 26 (2005) 2115-2120. DOI:10.1016/j.biomaterials.2004.06.013 |
| [2] |
J. Lu, C. Liu, P. Wang, et al., Biomaterials 62 (2015) 176-187. DOI:10.1016/j.biomaterials.2015.05.046 |
| [3] |
C. Monterrubio, G. Pascual-Pasto, F. Cano, et al., Biomaterials 79 (2016) 69-78. DOI:10.1016/j.biomaterials.2015.11.055 |
| [4] |
C.L. Gigliotti, R. Minelli, R. Cavalli, etal., J.Biomed. Nanotechnol. 12 (2016) 114-127. DOI:10.1166/jbn.2016.2144 |
| [5] |
C.G. Begley, L.M. Ellis, Nature 483 (2012) 531. DOI:10.1038/483531a |
| [6] |
M.Y. Huang, L.L. Zhang, J. Ding, et al., Chin. Med. 13 (2018) 35. DOI:10.1186/s13020-018-0192-y |
| [7] |
I.I. Skvortsova, V. Kumar, Curr. Med. Chem. 24 (2017) 4727-4728. |
| [8] |
Y. Qu, B. Chu, K. Shi, J. Peng, Z. Qian, J. Biomed. Nanotechnol. 13 (2017) 1598-1618. DOI:10.1166/jbn.2017.2475 |
| [9] |
R. Omar, Y.L. Bardoogo, E. Corem-Salkmon, B. Mizrahi, J. Control. Release 257 (2017) 76-83. DOI:10.1016/j.jconrel.2016.09.025 |
| [10] |
W. Ma, H Su, A.G. Cheetham, et al., J. Control. Release 263 (2017) 102-111. DOI:10.1016/j.jconrel.2017.01.015 |
| [11] |
W. Fan, B. Shen, W. Bu, et al., Chem. Sci. 6 (2015) 1747-1753. DOI:10.1039/C4SC03080J |
| [12] |
H. Wu, S Zhang, J. Zhang, et al., Adv. Funct. Mater. 21 (2011) 1850-1862. DOI:10.1002/adfm.201002337 |
| [13] |
N. Lei, C. Gong, Z. Qian, et al., Nanoscale 4 (2012) 5686-5693. DOI:10.1039/c2nr30731f |
| [14] |
H. Wang, G. Agrawal, L. Tsarkova, X. Zhu, M. Möller, Adv. Mater. 25 (2012) 1017-1021. |
| [15] |
T. Yu, A. Malugin, H. Ghandehari, ACS Nano 5 (2011) 5717-5728. DOI:10.1021/nn2013904 |
| [16] |
B. Ganguly, D.K. Srivastava, J. Biomed. Nanotechnol. 4 (2008) 457-462. DOI:10.1166/jbn.2008.010 |
| [17] |
J.H. Jung, K. Yoshida, T. Shimizu, Langmuir 18 (2002) 8724-8727. DOI:10.1021/la020594e |
| [18] |
Y. Zhang, G. Zhou, B. Sun, et al., Chem. Commun. 50 (2014) 2907-2909. DOI:10.1039/c3cc49511f |
| [19] |
J. Fan, Z. Xie, X. Teng, Y. Zhang, Chin. Chem. Lett. 28 (2017) 1104-1110. DOI:10.1016/j.cclet.2016.11.025 |
| [20] |
M. Zhang, L. Jiang, J. Biomed. Nanotechnol. 12 (2016) 1975-1986. DOI:10.1166/jbn.2016.2290 |
| [21] |
Q. He, J. Shi, Adv. Mater. 26 (2014) 391-411. DOI:10.1002/adma.201303123 |
| [22] |
N. Suzuki, S. Kiba, Y. Yamauchi, Mater. Lett. 65 (2011) 544-547. DOI:10.1016/j.matlet.2010.10.027 |
| [23] |
J. Lu, M. Liong, Z. Li, et al., Small 6 (2010) 1794-1805. DOI:10.1002/smll.201000538 |
| [24] |
B. Cheng, H. He, T. Huang, et al., J. Biomed. Nanotechnol. 12 (2016) 435-449. DOI:10.1166/jbn.2016.2195 |
| [25] |
Y. Zhu, J. Shi, W. Shen, et al., Angew. Chem. Int. Ed. 117 (2005) 5213-5217. |
| [26] |
J. Liu, Z. Luo, J. Zhang, et al., Biomaterials 83 (2016) 51-65. DOI:10.1016/j.biomaterials.2016.01.008 |
| [27] |
I.I. Slowing, J.L. Vivero-Escoto, C.W. Wu, et al., Adv. Drug Deliv. Rev. 60 (2008) 1278-1288. DOI:10.1016/j.addr.2008.03.012 |
| [28] |
J.L. Paris, M.V. Cabañas, M. Manzano, et al., ACS Nano 9 (2015) 11023-11033. DOI:10.1021/acsnano.5b04378 |
| [29] |
D. Dréau, L.J. Moore, M.P. Alvarez-Berrios, et al., J. Biomed. Nanotechnol. 12 (2016) 2172-2184. DOI:10.1166/jbn.2016.2318 |
| [30] |
J. Liu, Z. Luo, J. Zhang, et al., Biomaterials 83 (2016) 51-65. DOI:10.1016/j.biomaterials.2016.01.008 |
| [31] |
F. Tang, L. Li, D. Chen, Adv. Mater. 24 (2012) 1504-1534. DOI:10.1002/adma.201104763 |
| [32] |
J. Lu, M. Liong, J.I. Zink, et al., Small 3 (2007) 1341-1346. |
| [33] |
S.K. Sweeney, Y. Luo, M.A. O'Donnell, J.G. Assouline, J. Biomed. Nanotechnol. 13 (2017) 232-242. DOI:10.1166/jbn.2017.2339 |
| [34] |
D. Wang, J. Huang, X. Wang, et al., Biomaterials 34 (2013) 7662-7673. DOI:10.1016/j.biomaterials.2013.06.042 |
| [35] |
D. Borisova, H. Möhwald, D.G. Shchukin, ACS Nano 5 (2011) 1939-1946. DOI:10.1021/nn102871v |
| [36] |
Z. Li, J.C. Barnes, A. Bosoy, et al., Chem. Soc. Rev. 41 (2012) 2590-2605. DOI:10.1039/c1cs15246g |
| [37] |
Y. Yu, J. Duan, Y. Yu, Y. Li, Z.J. Sun, Biomed. Nanotechnol. 13 (2017) 485-499. DOI:10.1166/jbn.2017.2351 |
| [38] |
L.J. Van't Veer, H. Dai, M.J. van de Vijver, Y.D. He, Nature 415 (2002) 530. DOI:10.1038/415530a |
| [39] |
K. Michailidou, S. Lindström, J. Dennis, et al., Nature 551 (2017) 92. DOI:10.1038/nature24284 |
| [40] |
D. Carol, M. Jiemin, B. Leah, J. Ahmedin, CA Cancer J. Clin. 64 (2014) 52-62. DOI:10.3322/caac.21203 |
| [41] |
S.A. Narod, Rev. Clin. Nat., Oncol. 9 (2012) 460. |
| [42] |
Y. Qu, B.Y. Chu, J.R. Peng, et al., NPG Asia Mater. 7 (2015) e207. DOI:10.1038/am.2015.83 |
| [43] |
A. Wöckel, R. Wolters, T. Wiegel, et al., Ann. Oncol. 25 (2014) 628-632. DOI:10.1093/annonc/mdt584 |
| [44] |
S. Sahu, N. Sinha, S.K. Bhutia, M. Majhi, S. Mohapatra, J. Mater. Chem. B 2 (2014) 3799-3808. DOI:10.1039/C3TB21669A |
| [45] |
M.H. Chan, H.M. Lin, Biomaterials 46 (2015) 149-158. DOI:10.1016/j.biomaterials.2014.12.034 |
| [46] |
L. Yu, Y. Chen, H. Chen, Chin. Chem. Lett. 28 (2017) 1841-1850. DOI:10.1016/j.cclet.2017.05.023 |
| [47] |
J. Peng, L. Zhao, X. Zhu, et al., Biomaterials 34 (2013) 7905-7912. DOI:10.1016/j.biomaterials.2013.07.027 |
| [48] |
L. Pan, J. Liu, Q. He, J. Shi, Adv. Mater. 26 (2014) 6742-6748. DOI:10.1002/adma.v26.39 |
| [49] |
W. Xiong, H. Zhou, C. Zhang, H. Lu, Chin. Chem. Lett. 28 (2017) 2125-2128. DOI:10.1016/j.cclet.2017.09.019 |
| [50] |
R. Orecchia, Rev. Clin. Nat., Oncol. 11 (2014) 382. |
| [51] |
Y. Hao, J. Meng, S. Wan, Chin. Chem. Lett. 28 (2017) 2085-2091. DOI:10.1016/j.cclet.2017.10.019 |
| [52] |
K. Shi, Y.L. Wang, Y. Qu, et al., Sci. Rep. 6 (2016) 19077. DOI:10.1038/srep19077 |
 2018, Vol. 29
2018, Vol. 29 
