b School of Pharmacy, Fudan University, Key Laboratory of Smart Drug Delivery of MOE, Shanghai 201203, China;
c School of Pharmacy, Guilin Medical College, Guilin 541000, China;
d Health School Attached to Shanghai University of Medicine & Health Sciences, Shanghai 200237, China
Oral absorption is the most popular administration route due to good patients' compliance, safety and convenience. However, a large amount of drugs are difficult to exert optimal effect by oral delivery due to poor solubility or permeability [1, 2]. Nowadays, nanoparticle is considered as an effective carrier to improve the oral bioavailability of most drug molecules [3, 4]. Firstly, nanoparticles are able to protect drug molecules from degradation by numerous enzymes in gastrointestinal (GI) tract [2]. In addition, nanoparticles disperse throughout the intestine with gastric emptying after oral administration, which produces tremendous absorption areas [5]. Most of biodegradable nanoparticles are degraded by enzymes in GI lumens, such as liposomes and lipid nanoparticles [6, 7]. The components resulting from degradation would reconstitute to be mixed micelles together with endogenous bile salts and phospholipids, which is capable of enhanced absorption [7]. However, some nanoparticles are relatively stable in GI tract, such as polystyrene or inorganic nanoparticles [8, 9]. Many papers consider the non-biodegradable nanoparticles could improve the cellular uptake and transport [10-12]. After cellular uptake, nanoparticles could be decomposed in lysosome and release drugs into the cell or excreted out of cell from basolateral side [13], which contributes to enhanced absorption. However, there is lack of attentions on exocytosis towards apical side.
Nanoparticles internalizing into epithelia would undergo different traffic processes. Some nanoparticles are degraded in cells and release drug molecules which subsequently are transported across basolateral membrane into circulation [14], while rare intact nanoparticles could penetrate through basolateral membrane [15]. However, many researches have demonstrated that nanoparticles were taken up easilybut hard tobe transported across cell monolayer [16]. There is rare evidence to prove that a large amount of nanoparticles are able to transport across intestinal epithelia integrally [15]. Therefore, other two possible pathways are also important for nanoparticles after cellular uptake, including staying inside cells or exocytosis towards apical side. It is possible to show cytotoxicity if nanoparticles stay inside cells for a long period with no degradation [17]. Moreover, exocytosis towards apical side is a disadvantage for improving absorptionor treatment of intestinal diseases. Therefore, it is necessary to elucidate the possible fate of nanoparticles after cellular uptake.
The exocytosis of nanoparticles in endothelial cells has been elucidated in many papers, which illustrates that the exocytosis is highly dependent on size [18-20]. However, the intestinal epithelia are completely different from endothelial cells [21]. The epithelia include apical and basolateral sides which are highly different in biological characteristics. Therefore, it is extraordinary important for understanding the influence of nanoparticles characteristics on oral absorption by elucidating the bidirectional exocytosis in epithelia.
Our previous study illustrated that the uptake of polystyrene nanoparticles in Caco-2 cell was shape-dependent [22]. More nanorods were internalized into Caco-2 cells compared to their spherical or discal counterparts. But the influence of shape on intracellular fate of nanoparticles has not been well understood. In this paper, we attempt to clarify the in vivo behaviors of different shaped nanoparticles after cellular uptake, including exocytosis towards apical or basolateral sides, and retention in cells, which will further provide fundamental knowledge for oral application of different shaped nanoparticles.
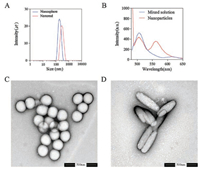
Nanorods were produced by stretching nanospheres which were obtained from market according to previous reports [8, 22]. The labeled size of nanospheres is 200 nm and size determined by dynamic light scattering (DLS) is about 180 nm as shown in Fig. 1A. The aspect ratio of nanorods was fixed at 2:1 in stretching. Hence, the size of nanorods is significantly different in length and width.
|
Download:
|
| Fig. 1. The particle distribution of nanospheres and nanorods determined by Zetasizer (A); the fluorescent spectrum of mixed solution (tetrahydrofuran solution of DiI and DiO) and nanoparticles loading DiI and DiO at a ratio of 1:1 (B); TEM images of nanospheres (C) and nanorods (D) | |
DLS only provides a Z-average size based on spherical model, hence the size of nanorods was determined as 300 nm by DLS measurement. However, the size of length is nearly 400 nm while width is about 100 nm, which was clearly observed by transmission electron microscopy (TEM) (Fig. 1D). Meanwhile, the TEM images (Figs. 1C and D) also showed nanospheres and nanorods were quite uniform in size distribution. Though the size of nanorods was significant different with that of nanospheres, the nanorods was obtained by stretching nanospheres. In fact, the differences between nanorods and nanospheres are only caused by the change of shape. Thus, these nanoparticles are the optimal objects to evaluate the shape effect. In order to quantify and observe the intact nanoparticles, both nanoparticles were labeled with DiO and DiI which were able to result in fluorescence resonance energy transfer (FRET) effect upon loading into nanoparticles simultaneously. Fig. 1B showed there was no peak at 565 nm (DiI emission wavelength) in mixed solution of DiI and DiO upon excitation at 484 nm (DiO excitation wavelength), while the peak at 565 nm occurred when DiI and DiO were loaded into nanoparticles together, which demonstrated the occurrence of FRET effect. The behaviors of intact nanoparticles are able to be tracked by FRET probes because the FRET fluorescence can be detected only when the nanoparticles maintain intact [8]. Once the nanoparticles are destroyed, the FRET effect immediately disappears because the distance of two probes exceeds far beyond 10 nm. Therefore, this study employed the FRET probes (DiI and DiO) to label nanospheres and nanorods for elucidating the intracellular behaviors accurately. The fluorescent intensity of nanospheres and nanorods is similar after loading the FRET probes, which are 1.12 ×109 and 1.08 × 109 [p/s]/[μW/cm2] by IVIS spectrum respectively.
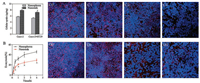
Caco-2 cells have been extensively used as an in vitro model of intestinal epithelia due to similar properties. In addition, the intestinal epithelia are covered by mucus layers protecting against bacterial infection [23]. The mucus is secreted by goblet cells which are substituted by HT29-MTX in vitro [24]. Therefore, single Caco-2 cell model and co-culture model of Caco-2 and HT29-MTX were developed to evaluate the uptake, transport and exocytosis of different shaped nanoparticles. Fig. 2A shows nanorods were taken up by both Caco-2 cells and co-culture models in greater amount than nanospheres. However, the uptake of nanoparticles in co-culture model decreases significantly compared to single Caco-2 cells, which could be ascribed to the impediment of mucus layer. After 2 h of uptake, nanoparticles were removed and the cells were washed by cold D-hank's buffer for three times. And then, the fresh HBSS was added in cells for exocytosis of nanoparticles. Nanoparticles were detected in fresh HBSS and the amount increased with the extension of time. Fig. 2B shows the exocytosis rate in Caco-2 cells before 30 min was very fast and slowed down after 1 h, and the exocytosis rate of nanospheres was much faster than that of nanorods. There were over 40% of nanospheres exocytosed from Caco-2 cells within 4 h. The similar trends were observed in co-culture models (Figs. S1 and S2 in Supporting information), but with smaller amount of exocytosed nanoparticles in total compared with single Caco-2 cells.
|
Download:
|
| Fig. 2. The cellular uptake of nanospheres and nanorods in Caco-2 cells and co-culture model of Caco-2 and HT29-MTX (A); The exocytosis percent of nanospheres and nanorods in Caco-2 cells at different time intervals (B); CLSM images of nanoparticles in Caco-2 cells: C0~C3 are images of cellular uptake, exocytosis of nanospheres at 15 min, 30 min and 2 h, respectively; D0~D3 are for nanorods. * Represents significant difference compared to nanospheres (P < 0.05) | |
Nanoparticles are taken up by endocytosis mediated via transport proteins, such as caveolin or clathrin, which is nonspecific [25]. Nanorods contact with cells in multi-orientation. If they contact with cells horizontally, nanorods would exert higher endocytosis effect [26, 27]. However, the contact area of nanospheres with cells is limited and unchanged in various directions. Most nanoparticles internalized into cells may suffer from degradation in lysosomes first and then escape into cytoplasma [28]. However, polystyrene is a non-biodegradable polymer which is rarely degraded in lysosomes [29], hence the polystyrene nanoparticles should exist as intact particles within the cells. That is to say, the fluorescent signals detected in cells were able to represent the intact nanoparticles as shown in Fig. 2 (C0~C3 and D0~D3). 2 h after exocytosis, the fluorescent intensity of nanospheres (Fig. 2C3) in cells was far lower than that of nanorods (Fig. 2D3). Most papers considered that the exocytosis of nanoparticles was mediated by microtubule [30, 31]. Nanospheres are easier to enter into microtubule than nanorods which were hindered by their specific shape. The rod-like shape led to the longterm retention of particles in cells, which could produce additional toxicity or be used to treat diseases inside cells.
Despite a large amount of uptake for both nanoparticles, only after transport acrossintestinal epitheliado the nanoparticles really contribute to improving absorption. Though the transport of nanorods across monolayer was a little bit more than nanospheres, the total transport was less than 2% (Figs. 3A1 and B1). Furthermore, the mucus layer in co-culture model of Caco-2 and HT29-MTX also impeded the transport of both nanoparticles. After 4 h of transport experiments, the nanoparticles were removed absolutely, and then the cell monolayers were washed by cold HBSS for three times. The fresh HBSS were added in apical (AP) and basolateral (BL) chambers for exocytosis towards AP and BL sides, respectively. Interestingly, more nanorods were excreted from BL side (Figs. 3A3 and B3) while more nanospheres were exocytosed from AP side (Figs. 3A2 and 3B2), which implied that the exocytosis mechanisms at AP and BL membranes were absolutely different. After 4 h of exocytosis, the monolayers were observed by CLSM, which showed more nanorods (Fig. 3D) stayed in cells compared to nanospheres (Fig. 3C). The similar results were obtained from co-culture monolayer of Caco-2 and HT29-MTX (Fig. S3 in Supporting information).

|
Download:
|
| Fig. 3. The percent of nanospheres and nanorods transported across Caco-2 monolayer (A1) and co-culture monolayer of Caco-2 and HT29-MTX (B1); The exocytosis of nanospheres and nanorods towards AP side in Caco-2 monolayer (A2) and co-culture monolayer (B2); The exocytosis towards BL side in Caco-2 monolayer (A3) and co-culture monolayer (B3); The CLSM images of nanospheres (C) and nanorods (D) after 4 h of exocytosis. * Represents significant difference compared to nanospheres (P < 0.05) | |
It has been proven that the nanoparticles were difficult to transport through epithelia in a great amount. In fact, most biodegradable nanoparticles enhance oral absorption by increasing dispersity or stability of drugs in GI tract, or by improving the apparent dissolution of poorly soluble drugs. The contribution of intact nanoparticles absorption to bioavailability was even negligible [32]. In contrast to transport, epithelial uptake of nanoparticles is tremendous, which leads to a viewpoint of "easy entry and hard across" for polymeric nanoparticles [16]. In this paper, this point of view was clarified again and we also demonstrated that most of nanoparticles were exocytosed from cells very fast. In addition, the shape of nanoparticles was found to be a critical factor influencing the exocytosis of nanoparticles. More nanorods stayed in the cells for a long time compared to nanospheres. Hence, the shape of nanoparticles should be intently considered in oral drug delivery because the shape could influence the therapeutic effects, toxicity or oral absorption.
In conclusion, the shape effect on exocytosis of nanoparticles were illustrated by cellular uptake and transport models.Both nanoparticles were taken upin agreat amount, butonlyless than 2% of nanoparticles were transported across epithelial monolayers. Most of nanoparticles were excreted out of cells after uptake, however the exocytosis rate of nanorods were slower than that of nanospheres significantly. Therefore, nanorods showed longer retention time in cells in comparison to nanospheres. Interestingly, nanoparticles' shape showed opposite effects on exocytosis towards AP and BL. Our findings provide an important direction for designing oral nanoparticles, but more distinct mechanisms should be further elucidated.
AcknowledgmentsThis work was financially supported by Science and Technology Commission of Shanghai Municipality (No. 18ZR1404100), Shanghai Pujiang Program (No. 18PJD001) and the National Natural Science Foundation of China (Nos. 81573363 and 81690263). Jie Zhuang would like to thank the financial support of the Youth Teacher Training Program of Shanghai Municipal Education Commission (No. ZZJKYX16011) and Shanghai Municipal Commission of Health and Family Planning (No. 20164Y0213).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.10.012.
| [1] |
H. Mu, R. Holm, A. Müllertz, Int. J. Pharm. 453 (2013) 215-224. DOI:10.1016/j.ijpharm.2013.03.054 |
| [2] |
E. Moroz, S. Matoori, J.C. Leroux, Adv. Drug Deliv. Rev. 101 (2016) 108-121. DOI:10.1016/j.addr.2016.01.010 |
| [3] |
K. Wang, Y. Feng, S. Li, et al., J. Biomed. Nanotech. 14 (2018) 1806-1815. DOI:10.1166/jbn.2018.2618 |
| [4] |
Y. Chen, Y. Jiao, Y. Ge, et al., J. Biomed. Nanotech. 13 (2017) 1147-1157. DOI:10.1166/jbn.2017.2403 |
| [5] |
E.M. Pridgen, F. Alexis, O.C. Farokhzad, Expert Opin. Drug Deliv. 12 (2015) 1459-1473. DOI:10.1517/17425247.2015.1018175 |
| [6] |
M. Cui, W. Wu, L. Hovgaard, et al., Int. J. Pharm. 489 (2015) 277-284. DOI:10.1016/j.ijpharm.2015.05.006 |
| [7] |
J. Qi, J. Zhuang, Y. Lu, et al., Drug Discov. Today 22 (2017) 166-172. DOI:10.1016/j.drudis.2016.09.024 |
| [8] |
D. Li, J. Zhuang, H. He, et al., ACS Appl. Mater. Interfaces 9 (2017) 42492-42502. DOI:10.1021/acsami.7b11821 |
| [9] |
C. Fu, T. Liu, L. Li, et al., Biomaterials 34 (2013) 2565-2575. DOI:10.1016/j.biomaterials.2012.12.043 |
| [10] |
G. Janer, E. Mas del Molino, E. Fernandez-Rosas, et al., Toxicol. Lett. 228 (2014) 103-110. DOI:10.1016/j.toxlet.2014.04.014 |
| [11] |
A. Beloqui, A. des Rieux, V. Préat, Adv. Drug Deliv. Rev. 106 (2016) 242-255. DOI:10.1016/j.addr.2016.04.014 |
| [12] |
Y.P. Jia, B.Y. Ma, X.W. Wei, et al., Chin. Chem. Lett. 28 (2017) 691-702. DOI:10.1016/j.cclet.2017.01.021 |
| [13] |
A.R. Neves, J.F. Queiroz, S.A.C. Lima, et al., J. Colloid Interface Sci. 463 (2016) 258-265. DOI:10.1016/j.jcis.2015.10.057 |
| [14] |
S. Simovic, Y. Song, T. Nann, et al., Nanomedicine 11 (2015) 1169-1178. DOI:10.1016/j.nano.2015.02.016 |
| [15] |
X. Hu, W. Fan, Z. Yu, et al., Nanoscale 8 (2016) 7024-7035. DOI:10.1039/C5NR07474F |
| [16] |
B. He, P. Lin, Z. Jia, et al., Biomaterials 34 (2013) 6082-6098. DOI:10.1016/j.biomaterials.2013.04.053 |
| [17] |
R. Sakhtianchi, R.F. Minchin, K.B. Lee, et al., Adv. Colloid Interface Sci. 201 (2013) 18-29. |
| [18] |
N. Oh, J.H. Park, Int. J. Nanomed. 9 (2014) 51-63. |
| [19] |
L. Shang, K. Nienhaus, G.U. Nienhaus, Int. J. Nanobiotechnol. Pharm. 12 (2014) b26. DOI:10.1186/s12951-014-0026-8 |
| [20] |
H. Kim, J.H. Choi, S.R. Kang, et al., J. Biomed. Nanotech. 12 (2016) 536-545. DOI:10.1166/jbn.2016.2189 |
| [21] |
S.M. Thomasy, V.K. Raghunathan, M. Winkler, et al., Acta Biomater. 10 (2014) 785-791. DOI:10.1016/j.actbio.2013.09.025 |
| [22] |
A. Banerjee, J. Qi, R. Gogoi, et al., J. Control. Release 238 (2016) 176-185. DOI:10.1016/j.jconrel.2016.07.051 |
| [23] |
H. Li, J.P. Limenitakis, T. Fuhrer, et al., Nat. Comm. 6 (2015) 8292-8303. DOI:10.1038/ncomms9292 |
| [24] |
M.A. Engevik, R.S. Fultz, B.P. Ganesh, et al., FASEB J. 30 (2016) 1017.4. |
| [25] |
E. Blanco, H. Shen, M. Ferrari, Nat. Biotechnol. 33 (2015) 941-951. DOI:10.1038/nbt.3330 |
| [26] |
S. Dasgupta, T. Auth, G. Gompper, Nano Lett. 14 (2014) 687-693. DOI:10.1021/nl403949h |
| [27] |
A.C. Anselmo, S. Kumar, V. Gupta, et al., Biomaterials 68 (2015) 1-8. DOI:10.1016/j.biomaterials.2015.07.043 |
| [28] |
Y. Xu, Y. Zheng, L. Wu, et al., ACS Appl. Mater. Interfaces 10 (2018) 9315-9324. DOI:10.1021/acsami.8b00507 |
| [29] |
B.T. Ho, T.K. Roberts, S. Lucas, Crit. Rev. Biotechnol. 38 (2018) 308-320. DOI:10.1080/07388551.2017.1355293 |
| [30] |
Y. Wang, C. Yao, C. Li, et al., Nanoscale 7 (2015) 13105-13115. DOI:10.1039/C5NR03269E |
| [31] |
L. Ding, X. Zhu, Y. Wang, et al., Nano Lett. 17 (2017) 6790-6801. DOI:10.1021/acs.nanolett.7b03021 |
| [32] |
P. Lundquist, P. Artursson, Adv. Drug Deliv. Rev. 106 (2016) 256-276. DOI:10.1016/j.addr.2016.07.007 |
 2018, Vol. 29
2018, Vol. 29 
