b College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325027, China
A low-molecular-weight gel with dual pH and glucose sensitivity was designed as the gate controller for mesoporous silica nanoparticles (MSNs) to fabricate a smart drug delivery system. The smart gel caped MSNs could control the antidiabetic drug release via the detection of glucose and pH levels.
The application of MSNs with stimuli-responsive gate has attracted considerable attention as drug carriers, due to the rapid response to physical, chemical or biochemical stimuli [1-4]. The capped MSNs exhibit significant potencies in nano-objects for the drug delivery via biological pathways. Most of the stimuli responsive MSNs were gated by covalent bond surface modification of polymes, organic molecules, biological macromolecules and inorganic nanoparticles as "guards" [5-8]. The guard valves controlled the drug release via the stimuli response to light, pH, temperature and biological molecules [9-11]. However, the covalent bond modifications of MSNs with proteins, enzymes and genes would damage the activity of the biomolecules [12, 13], and the "gates" covalent bonded on the surface of MSNs would affected the drug loading efficiency [14-18].
Different from the covalent bond modification, weak noncovalent interactions such as coordination bond, hydrogen bond, van der Waals force and host-guest interaction could drive the selfassembly of "gate" molecules to avoid the limitations of covalent bonded "gates" [19]. Low molecular weight gels as soft matters have attracted attention as "gates" for MSNs in recent years [20-22]. The LMWG could not only coat on the surface of MSNs after drug loading but also penetrate in the capillary to maintain sustaining release of therapeutics.
Glucose-responsive materials have the capability to regulate insulin or hypoglycemic drug release in responding to the change of glucose level in blood, which are used in treating diabetes mellitus with worldwide health concern [23-25]. Although glucose-responsive polymers have been reported, the rapid response to glucose at physiological conditions is still craved [26, 27]. In this paper, we developed a dual pH and glucose responsive low molecular weight gelator as the gate to fabricate MSNs based antidiabetic drug delivery system, the hypoglycemic agents loaded MSNs were capped with the LMWG to regulate the delivery of hypoglycemia.
A pH and glucose-sensitive LMWG was synthesized (Scheme S1 in Supporting information). The hypoglycemic drug of phenformin was absorbed physically in mesoporous silica, the drug-loaded mesoporous silica nanoparticles were gated with LMWG using non-covalent interactions to block the mesopores, the mesoporous channels would open when the gel was responsive to the external stimuli of pH and glucose to release drug (Fig. 1).
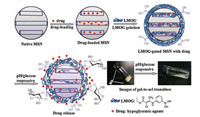
|
Download:
|
| Fig. 1. Schematic diagram of the fabrication of dual pH and glucose sensitive LMWG gated MSNs drug delivery system. | |
The in vitro cytotoxicity of the gel was evaluated using NIH/3 T3 fibroblasts (Fig. S1A in Supporting information). The cell viabilities were beyond 90% for the gel extracts, even the concentration of gelator was as high as 5 mg/mL. Confocal laser scanning microscopy was further used to explore the alive cells in the gels (Fig. S1B in Supporting information). The green fluorescence demonstrated that the NIH/3 T3 cells could live and migrate from surface to bulk of gels [28]. These results demonstrated that the gels were biocompatible. The cytotoxicity of LMWG gated MSNs was investigated as shown in Fig. S2 in Supporting information. The cell viabilities were beyond 90% for all the extracts of gel gated MSNs (Fig. S2A), and the NIH/3 T3 cells could live and migrate in the bulk of gel-gated MSNs (Fig. S2B). These results demonstrated that the LMWG gated MSNs were biocompatible too.
The maximum drug loading content (DLC) and encapsulation efficiency (EE) of MSNs were 46.2% and 91.4% when the concentration of phenformin was 8 mg/mL. The drug-loaded MSNs were characterized by FT-IR spectrum, as shown in Fig. S3A in Supporting information. The broad band stretch around 3450 cm-1 was attributed to O—H stretching vibrations, the bands at 1634 cm-1 and 1562 cm-1 were assigned to C=N symmetric stretching vibration of phenformin, the band at 1090 cm-1 was SiO-Si antisymmetric stretching vibration, the bands at 800 cm-1 and 466 cm-1 were assigned to Si—O symmetric stretching vibration. The results demonstrated that phenformin was successfully loaded in mesoporous MSNs. In the FT-IR spectrum of LMWG gated MSNs, typical bands of gelator at 3414 cm-1 (O—H), 3259 cm-1 (N—H), and those of mesoporous silica at 3452 cm-1 (O—H), 1091 cm-1 (Si—O—Si), Si—O (801 cm-1), and 466 cm-1 (Si—O) were found as shown in Fig. S3.
SEM image of the air-dried gel (Fig. 2b) indicated that the gel was consisted of nanofibers (50-100 nm wide) to form threedimensional network. The MSNs were monodisperse with uniform diameter (100 nm) in spherical shape (Figs. 2c and d). To LMWG gated MSNs, the gel was observed both on the surface and in the capillary of MSNs (Figs. 2e and f). In high magnified TEM images (Fig. S4 in Supporting information), less or no mesopores were observed in the enlarged TEM images of LMWG gated MSNs. For native MSNs, there was well-arranged mesoporous in the image. The results implied that the LMWG was filled in the mesopores of MSNs. The particle size of LMWG gated MSNs increased to 133 nm, with the comparison of the native MSNs size 106 nm, it demonstrated that the LMWG was coated on the surface of MSNs as the gate (Fig. S5 in Supporting information).

|
Download:
|
| Fig. 2. The morphology of LMWG gated MSNs: (a) gelator, (b) SEM image of xeogel, (c) TEM and (d) SEM images of mesoporous silica nanoparticles, (e) TEM and (f) SEM images of LMWG gated MSNs. | |
The absorption capability of LMWG gated MSN was evaluated. There was a hysteresis loop for native xeogel, it implied the adsorption and desorption curves were different under medium pressure (Fig. 3a). Native MSNs belonged to a type Ⅳ adsorption/ desorption (Fig. 3b), single molecule layer adsorption and subsequently multilayer adsorption were appeared under relatively low pressure, the capillary condensation and a jump in adsorption isothermal line were appeared when the pressure was higher. There was a hysteresis loop for gel gated MSNs, which was similar to that of native MSNs under high pressure, indicating that the gel gated MSNs still had the performance of native MSNs (Fig. 3c). The BET specific surface area and t-plot external surface area of these nanoparticles were available. BET surface area of native gel was low, which was only 3.1 m2/g. Mesoporous silica showed a larger specific surface area up to 835.9 m2/g, which provided a large capacity for drug attachment. When gated with gel, the specific surface area of the MSNs decreased greatly to 12.2 m2/g, which illustrated that the gel blocked the mesopores effectively. After the release of phenformin, the MSNs were free from gel and the specific surface area increased (Fig. 3d).

|
Download:
|
| Fig. 3. The BET of (a) gelator, (b) native MSNs, (c) LMWG gated MSNs with drug loading (50 mg gelator with 25 mg drug-loaded MSN), (d) LMWG gated MSNs after drug release. | |
The pH and glucose sensitivities of the gel gated MSNs were investigated. At a low concentration of 2.1 mg/mL (critical concentration of venous plasma glucose for diabetes), the gel gated native MSN showed a great sensitivity, glucose response of the gel decreased with the increasing concentration of native MSN, as shown in Fig. 4A. In vitro release study of phenformin in gel gated MSN was carried out in glucose solution with different concentrations (0, 1.0, 2.1, 5.0, 10.0, 30.0 mg/mL), as shown in Fig. 4B. Phenformin-loaded MSN was used as the control. There was a burst release of 87.0% in 35.5 h, and the drug release reached equilibrium, a total of 90.9% drug was released after 116 h. When phenformin loaded MSNs was capped with gel, the release rate was controllable. Nearly no phenformin was released from gel gated MSNs with no glucose or low glucose concentration of 1.0 mg/mL even after 116 h. The release profile showed glucose sensitivity with a low glucose concentration of 2.1 mg/mL (critical concentration of venous plasma glucose for diabetes). The drug release rate increased with increasing glucose concentration. 31.5% of the loaded phenformin was released in the glucose solution of 2.1 mg/ mL for 116 h, and the cumulative release rates of phenformin were 36.7% in the glucose solution of 5.0 mg/mL, 48.2% in the glucose solution of 10.0 mg/mL, and 71.4% in the glucose solution of 30.0 mg/mL, respectively.

|
Download:
|
| Fig. 4. Drug release profiles of dual pH and glucose responsive LMWGs gated MSNs: (A) glucose responsive dynamics of different gel/MSNs ratios in glucose solution (2.1 mg/mL), (B) commulative release of phenformin from LMWG gated MSNs in different glucose solutions, (C) pH responsive dynamics of the LMWG gated MSNs in PBS with different pH values, (D) cumulative release of phenformin from LMWG gated MSNs in PBS with different pH, the release profile of drug-loaded native MSN in PBS 7.4 was the control. All the gels were made of 20 mg gelator in 0.4 mL cyclohexane. | |
The pH-sensitive behavior of LMWG gated MSN swasevaluatedin Fig. 4C. The sensitivity was great in the solution with pH 12.8. The rapid increase in UV-absorbance suggested the increasing pHresponse rate at high pH. The in vitro release study of phenformin in LMWG gated MSNs were performed in phosphate buffer with different pH values (1.0, 4.0, 6.2, 7.4, 9.0, 11.0, 12.8), as shown in Fig. 4D. Phenformin loaded MSNs in PBS (pH 7.4) was used as the control. There was a burst release of 86.5% in 35.5 h, and the drug release reached equilibrium, total of 89.5% was released after 55.5 h. The gel capped MSNs exhibited controllable release behavior. Nearly no phenformin was released from gel gated MSNs in the pH values ranged from 6.2 to 9.0. The gel gated MSNs exhibited pH sensitivity in PBS with pH lower than6.2orhigher than9.0. A totalof 17.2%, 19.4%, 24.3% of the loaded phenformin were released after 55.5 h in PBS with pH values of 1.0, 4.0, 11.0, respectively. The greatest rate of 59.3% was obtained in PBS with pH value of 12.8. The high pH dependant response was probably attributed to the ionization of PBA, which accelerated the destruction of hydrogen-bonding to favor the gel-sol transition [29].
In conclusion, we fabricated a smart drug delivery system using a phenylboronic acid derivative based LMWG with dual pH and glucose sensitivity as the gate controller for MSNs. The in vitro cytotoxicity test demonstrated the gel was non-toxic and biocompatible. The LMWG played a good sealing effect both in filling the pores as well as coating on the surface of MSNs to control the drug release. The LMWG gated MSNs exhibited good performance of intelligent release of antidiabetic drugs due to the glucose and pH responsive gel gate. The research provides an effective strategy to fabricate noncovalent gel gated MSNs for smart drug release.
AcknowledgmentsThe authors thank the National Natural Science Foundation of China (No. 21672164), the Natural Science Foundation of Zhejiang Province (No. LY15B020001) for the financial support.
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.05.022.
| [1] |
E. Aznar, M. Oroval, L. Pascual, et al., Chem. Rev. 116 (2016) 561-718. DOI:10.1021/acs.chemrev.5b00456 |
| [2] |
Z.X. Li, J.C. Barnes, A. Bosoy, J.F. Stoddart, J.I. Zink, Chem. Soc. Rev. 41 (2012) 2590-2605. DOI:10.1039/c1cs15246g |
| [3] |
R. Bhat, A. Ribes, N. Mas, et al., Langmuir 32 (2016) 1195-1200. DOI:10.1021/acs.langmuir.5b04038 |
| [4] |
Y. Chang, X. Liu, Q. Zhao, et al., Chin. Chem. Lett. 26 (2015) 1203-1208. DOI:10.1016/j.cclet.2015.08.005 |
| [5] |
N. Song, Y.W. Yang, Chem. Soc. Rev. 44 (2015) 3474-3504. DOI:10.1039/C5CS00243E |
| [6] |
A.A. Hwang, J. Lu, F. Tamanoi, J.I. Zink, Small 11 (2015) 319-328. DOI:10.1002/smll.v11.3 |
| [7] |
L. Zhang, Y. Li, J.C. Yu, J. Mater. Chem. B 2 (2014) 452-470. DOI:10.1039/C3TB21196G |
| [8] |
Q. Zhang, Z. Ye, S. Wang, J. Yin, Chin. Chem. Lett. 25 (2014) 257-260. DOI:10.1016/j.cclet.2013.11.002 |
| [9] |
S. Baek, R.K. Singh, D. Khanal, et al., Nanoscale 7 (2015) 14191-14216. DOI:10.1039/C5NR02730F |
| [10] |
S.A. Mackowiak, A. Schmidt, V. Weiss, et al., Nano Lett. 13 (2013) 2576-2583. DOI:10.1021/nl400681f |
| [11] |
D. Wang, S. Wu, Langmuir 32 (2016) 632-636. DOI:10.1021/acs.langmuir.5b04399 |
| [12] |
S.W. Zhou, X.Z. Du, F.B. Cui, X.F. Zhang, Small 10 (2014) 980-988. DOI:10.1002/smll.201302312 |
| [13] |
J.G. Croissanta, D. Zhang, S. Alsaiari, et al., J. Control Release 229 (2016) 183-191. DOI:10.1016/j.jconrel.2016.03.030 |
| [14] |
Y. Zhang, C.Y. Ang, M. Li, et al., ACS Appl. Mater. Interfaces 7 (2015) 18179-18187. DOI:10.1021/acsami.5b05893 |
| [15] |
J. Lee, E. Oh, H. Yoon, et al., Nanoscale 8 (2016) 8070-8077. DOI:10.1039/C5NR09280A |
| [16] |
C.L. Zhu, C.H. Lu, X.Y. Song, H.H. Yang, X.R. Wang, J. Am. Chem. Soc. 133 (2011) 1278-1281. DOI:10.1021/ja110094g |
| [17] |
T. Chen, W. Wu, H. Xiao, et al., ACS Macro Lett. 5 (2016) 55-58. DOI:10.1021/acsmacrolett.5b00765 |
| [18] |
C. Yu, L. Qian, J. Ge, et al., Angew. Chem. Int. Ed. 55 (2016) 9272-9276. DOI:10.1002/anie.201602188 |
| [19] |
Y. Li, W. Xiao, K. Xiao, et al., Angew. Chem. Int. Ed. 124 (2012) 2918-2923. DOI:10.1002/ange.201107144 |
| [20] |
Y. Zhou, X. Li, Chin. Chem. Lett. 28 (2017) 1835-1840. DOI:10.1016/j.cclet.2017.04.033 |
| [21] |
B.O. Okesola, D.K. Smith, Chem. Soc. Rev. 45 (2016) 4226-4251. DOI:10.1039/C6CS00124F |
| [22] |
E.R. Draper, D.J. Adams, Chem. Commun. 52 (2016) 8196-8206. DOI:10.1039/C6CC03485C |
| [23] |
Z. Zou, D. He, L. Cai, et al., ACS Appl. Mater. Interfaces 8 (2016) 8358-8366. DOI:10.1021/acsami.5b12576 |
| [24] |
D. Shi, M. Ran, L. Zhang, et al., ACS Appl. Mater. Interfaces 8 (2016) 13688-13697. DOI:10.1021/acsami.6b02121 |
| [25] |
L. Tan, M. Yang, H. Wu, et al., ACS Appl. Mater. Interfaces 7 (2015) 6310-6316. DOI:10.1021/acsami.5b00631 |
| [26] |
L. Zhao, C. Xiao, L. Wang, G. Gaia, J. Ding, Chem. Commun. 52 (2016) 7633-7652. DOI:10.1039/C6CC02202B |
| [27] |
P. Diez, A. Sanchez, C. de la Torre, et al., ACS Appl. Mater. Interfaces 8 (2016) 7657-7665. DOI:10.1021/acsami.5b12645 |
| [28] |
S. Pan, S. Luo, S. Li, et al., Chem. Commun. 49 (2013) 8045-8047. DOI:10.1039/c3cc44767g |
| [29] |
C. Zhou, W. Gao, K. Yang, et al., Langmuir 29 (2013) 13568-13575. DOI:10.1021/la4033578 |
 2018, Vol. 29
2018, Vol. 29 

