b Beijing Key Laboratory of Ionic Liquids Clean Process, CAS Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
As people have paid more and more attention to the shortage of fossil energy and environmental protection, lithium ion batteries attracted much attention due to their meaningful application in renewable energy storage system. Lithium-ion batteries (LIB) have experienced growing acceptance in 3C electronics, electric vehicles, mass energy storages and military industry, etc., because of their high energy density, high power density, wide operating temperature, low self-discharge, no memory effect and environment-friendly advantages [1-4]. To further enhance the properties of LIBs, developing new electrodes materials and more efficient electrolytes systems to match the materials is very important. Recently, with the fast development of exploring new electrodes materials, electrolyte studies become the bottleneck for preventing the LIB from performance increasing. The key points for electrolytes studies including developing high voltage electrolyte [5], high safety electrolyte [6, 7], wide range temperature electrolyte [8], long lifetime electrolyte, etc. Among them, safety issues has always been one of the critical issues in electrolyte studies, because there are some security risks of LIB directly connected with electrolytes, such as thermal shock, overcharge, over discharge, short circuit, tend to have fire, blasting and other security risks [9-11]. In order to avoid the safety risks of LIB which mentions above, the researchers developed many approaches to solve the problem. For example, Xia et al. reported that by surface modification of NCA materials with FePO4 effectively improves the cycle and thermal stability of the material [12]. Huang et al. found improvements in the fire-extinguishing behavior of the cathode/ electrolyte mixture are achieved by using a pre-embedded phosphorus-based flame retardant [13]. The second is to modify the separator and electrode structures, when the temperature is too high, they can release some flame retardants [14]. The third way is optimization on the electrolyte formulation. In terms of electrolytes, there are two ways to do. One is to replace the traditional carbonates solvent with a higher melting point new solvent [15], and the other is to add some flame retardant additives into the tradition carbonate solvents [16]. The latter one is more convenient and low cost. A variety of literatures were reported on the study of flame retardants for LIB electrolyte, in accordance with the flame retardant elements, they can be divided into phosphorus flame retardant additives, halogen flame retardant additives, silicon flame retardant additives, composite flame retardant additives [17-20]. At present industry, the widely used flame retardants are halogen based flame retardants, such as PBDEs, HBCD and other BFRs, which have been shown to be harmful to the environment and our body [21, 22]. In this study, we studied three new types of flame retardants, they are methyl diethyl phosphonoacetate (MDPCT), triethyl2-fluoro-2-phosphonoacetate (TFPCT), Carbethoxy ethylidene triphenylphosphorane (CETPE). We studied the flammability, stability, and electrochemical properties of these three additives in the electrolyte to determine whether they are suitable as a lithium battery flame retardant or not.
The electrolyte used as the blank sample in this study was 1.0 mol/L lithium hexafluorophosphate (LiPF6) in ethylene carbonate (EC):dimethyl carbonate (DMC):diethyl carbonate (DEC) (1:1:1, v/v/v). Then adding a certain amount of MDPCT, TFPCT, CETPE, prepared as a weight ratio of 5%, 10%, 15% of the electrolyte. The prepared electrolyte was sealed and stored in a super-clean glove box. In this paper, the ternary materials of nickel-cobalt-aluminum (LiNi0.8Co0.15Al0.05O2, abbreviation NCA) are used as the cathode material, due to the NCA has the advantages of high capacity, good rate performance and good low-temperature performance [23]. The polyvinylidene fluoride (PVDF) was used as the binder. The electrode material, conductive agent, and binder were mixed at a mass ratio of 8:1:1. Then, an appropriate amount of N-methyl-pyrrolidone (NMP) was added, and the mixture was stirred for 12 h in a heated magnetic stirring. The resulting slurry was smoothly applied to the aluminum sheet in a dust-free room and rolled into a film electrode of about 0.06 mm to 0.10 mm thick, followed by drying in a vacuum oven at 130 ℃ for 24 h. Finally, CR2025 coin cells were then fabricated in the glove-box.
Self-extinguishing time (SET) experiment was conducted to test the effectiveness of the flame retardant according to the reported literature method [24].The experimental procedure is as follows. First, the raw material is prepared with glass wool into a batch of spheres with a radius of about 5 mm, weigh the quality and record it. Then, it was immersed in an electrolytic solution and weighed. The quality difference before and after immersion is the quality of glass wool absorbs the electrolyte. Finally, the cotton ball was placed on the front round wire, ignited using a gas ignition device, and the time taken to extinguish was recorded. To determine the heat release during electrolyte decomposition, differential scanning calorimetry (DSC1, Mettler-Toledo Corp.) of the electrolyte were conducted. DSC text is to identify the stability of the electrolyte. The scanning temperature was set at 25 ℃ to 400 ℃, and the scanning speed was 10 ℃/min. The cyclic voltammetry (CV, 2.8-4.6 V, 0.1 mV/s) and electrochemical impedance spectroscopy (EIS) measurements (100 kHz-10 mHz, 5 mV perturbation) were completed on electrochemical station (Metrohm Auto lab M204). Cyclic voltammetry (CV) measure the electrochemical stability of the electrolyte and the electrochemical viability of the electrode. We tested the assembled button batteries with electrochemical workstations, and the voltage is from 2.0 V to 4.3 V. Long term cycling performances of the electrodes in different flame retardant containing electrolytes were counted on a LAND (CT2001 A) cycler by testing in CR2025 coin cells of Li/NCA. The voltage is 2.8 V to 4.3 V, and the test current is 0.5C constant current charge and discharge.
In order to study the electrode/electrolyte interface, an accelerated cycling of the cells was performed prior to any measurements, which to verify whether the additive is involved in the formation of the battery CEI film. After we cycle with 100 cycles, we observed the morphology the cathode of cell by Scanning electron microscopy (SEM, Hitachi SU8020, Japan).
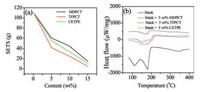
The flammability of the electrolytes with different additives were evaluated by testing the self-extinguish time (SET) [25]. The SET result was shown in Fig. 1a, we can clearly find that the flammability of the electrolyte is rapidly reduced as the concentration of the flame retardants increases. When the additives concentration reaches 15 wt%, the SET of the three electrolytes is less than 20 s/g. MDPCT, CETPE can generate phosphorus-containing free radicals, and TFPCT can generate phosphorus-containing and fluorine-containing free radicals. These free radicals can be combined with the H• and HO• radicals released by the burning electrolyte [26-28]. Thus, MDPCT, CETPE and TFPCT can prevent the burning of the electrolyte. From Fig. 1a, we can see that TFPCT has the best flame retardant effect; it should be attributed to the synergies of free radicals generated by phosphorus-containing and fluorine-containing groups that prevented the electrolyte from burning. The thermal stabilities of the blank electrolyte and the additives-containing electrolytes were further studied by differential scanning calorimetry (DSC) [29]. As shown in Fig. 1b, the blank electrolyte has two exothermic peaks which located at 110 ℃ and 181 ℃. Blank electrolyte + 5 wt% MDPCT and CETPE is safer than blank electrolyte because their exothermic peaks move to the right and peak area also smaller compared with blank electrolyte. From the uppermost curve, we can see that blank electrolyte + 5 wt% TFPCT have only one exothermic peak and the peak area is also smaller compared with other three curves, indicating its best thermal stability. We speculate that when the temperature rises, additives decompose and absorb heat, thereby reducing the heat generated by the decomposition of the electrolyte, increasing the stability of the electrolyte [30].
|
Download:
|
| Fig. 1. (a) Self-extinguishing time (SET) with various contents of MDPCT, TFPCT and CETPE in blank electrolyte. (b) DSC curves of blank electrolyte, blank electrolyte + 5 wt% CETPE, blank electrolyte + 5 wt% TFPCT and blank electrolyte + 5 wt% MDPCT. | |
Fig. 2 presents the electrochemical performance of Li/NCA halfcells in different electrolytes. In order to determine the optimum additive concentration, we selected 5 wt%, 10 wt%, 15 wt% of TFPCT flame retardant for research (Fig. 2a). From Fig. 2a, it can be seen that the initial discharge capacity of the cell with 5 wt% TFPCT remains over 180 mAh/g. 10 wt% TFPCT is 125 mAh/g, and 15 wt% is only 50 mAh/g after 90 cycles, respectively. We can conclude that 5 wt% of flame retardant has a good cycle performance, thus, in the following experiments, 5 wt% of flame retardant was chosen. In addition, to further investigate the three additives' effects on the performances of the NCA half cells, Fig. 2b shows the obtained results of the half cells based on blank electrolyte and three additive-containing electrolytes at the room temperature. As the number of cycles increases, the discharge capacity of all the four cells decreased, compared with the blank electrolyte, the cells containing 5 wt% MDPCT, 5 wt% CETPE and 5 wt% TFPCT showed better specific capacities retention. It was found that the cell of blank electrolyte decreased significantly and its discharge capacity less than 120 mAh/g after 100 cycles, however, the addition of three additives significantly improves the cycling performance, especially addition of TFPCT, the capacity retention of 94.03% is achieved for the NCA with the addition of 5 wt% TFPCT after 100 cycles at a current density of 0.5C, which is due to the synergistic effect of fluorine that better promoted the formation of SEI film [31]. The poor cycle performance is generally due to the increase of interface impedance or irreversible capacity during the charge and discharge process [32]. To clarify this, we investigate the electrochemical impedance of the battery before and after cycling (Figs. 2c and d). All the impedance reduced after cycles, which can be ascribed to the solid electrolyte interface (CEI) film formed during the process and induce a lower interfacial impedance [33]. And among the results of different additives, the most effective one is TFPCT, we speculated that TFPCT could form a more dense and uniform CEI film on the surface of the NCA electrode during cycle performances, so that the lithium ions can be freely transported while avoiding further contact between the electrolyte and the electrode material and lowering the interface impedance [34], which also confirmed by our SEM studies.

|
Download:
|
| Fig. 2. The electrochemical performance of Li/NCA half-cells in different electrolytes. (a) Cycle performances of Li/NCA half cells in blank electrolyte, blank electrolyte + 5 wt% TFPCT, blank electrolyte + 10 wt% TFPCT, blank electrolyte + 15 wt% TFPCT. (b) Cycle performances of Li/NCA cells in blank electrolyte, blank electrolyte + 5 wt% CETPE, blank electrolyte + 5 wt% TFPCT, and blank electrolyte + 5 wt% MDPCT, the current density is 0.5C. (c) EIS plots of Li/NCA cells in blank electrolyte and electrolytes with different additive before cycling. (d) EIS plots of Li/NCA cells in blank electrolyte and electrolytes with different additive after seven cycles. | |
The cyclic voltammograms (CVs) curves of Li/NCA cells by using blank electrolyte and additives-containing electrolytes are shown in Fig. 3. For the cell, the first curve is significantly different from the later curves. The oxidation peaks in the initial cycle were 3.83 V, 3.99 V and 4.19 V, corresponding to the reduction peaks at 3.70 V, 3.95 V and 4.16 V, respectively [35]. However, for the blank electrolytes group (Fig. 3a), the curve of the fourth has not overlapped, and the other curves of the cells with additives have been overlapped on the third and the fourth. It is noteworthy that the third and fourth curves have the highest degree of coincidence in the presence of TFPCT (Fig. 3c), and furthermore it has a higher peak current than the other three graphs (Figs. 3a, b and d). We speculate this is due to the formation a CEI film during charge and discharge, reducing the interface impedance which makes the battery more stable [36].

|
Download:
|
| Fig. 3. Cyclic voltammograms (CVs) curves: (a) Li/NCA cells in blank electrolyte, (b) blank electrolyte + 5 wt% CETPE, (c) blank electrolyte + 5 wt% TFPCT, (d) blank electrolyte + 5 wt% MDPCT. | |
The surface morphology of the NCA electrode cycled in blank electrolyte and additive-containing electrolytes were characterized by SEM. Fig. 4 presents the SEMimages ofNCA surface. The surfaceof the NCA electrode without the addition of additives has been destroyed, and a lot of cracks were formed after 100 cycles (Fig. 4a). The destroyed surface may lead to the lithium ion cannot migrate effectively, eventually, the battery discharge efficiency was reduced. By contrast, the structure of NCA electrode remains intact with additive-containing electrolytes (Figs. 4b-d). Furthermore, the TFPCT show best film-forming effect among all the three additives, which also keep in accordance with our above CV and EIS studies, this indicated that the three flame retardant-derived CEI layers relatively thick and uniform which effectively improves the stability of the interface and suppression of electrolyte decomposition. In addition, the existence of film can facilitate the migration of lithium ions and has a significant advantage of reducing interface resistance (Figs. 2c and d) and increasing discharge capacity (Fig. 2b). As a result, the electrochemical performances of the battery were enhanced. It is verified that adding flame retardant to electrolytes can obviously improve the electrochemical performance of the battery.

|
Download:
|
| Fig. 4. SEM images of the NCA electrode after 100 cycles: (a) blank electrolyte, (b) blank electrolyte + 5 wt% CETPE, (c) blank electrolyte + 5 wt% TFPCT, (d) blank electrolyte + 5 wt% MDPCT. | |
In this work, three new flame retardant additives were investigated to improve the safety of the NCA based lithium ion batteries. By adding different amounts of MDPCT, CETPE and TFPCT additives into the blank electrolytes, the SET time can be shortened efficiently, and the thermal stability of the additives-containing electrolytes increased remarkably. The electrochemical tests show that the addition of 5 wt% TFPCT can enhance the discharge capacity of NCA/Li half-cell, exhibiting a capacity retention as high as 92.2% at a current density of 0.5C after 100 cycle. The results from cyclic voltammograms, electrochemical impedance spectroscopy, scanning electron microscopy, and X-ray photoelectron spectroscopy demonstrate all the three flame retardant additives facilitates the formation of solid electrolyte interfaces, which can protect the NCA electrode under the high temperature and hinder the subsequent decomposition of the electrolyte, resulting in better cycling performance of the battery. Our results may useful for developing high safety NCA-based lithium ion batteries with high energy density.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21503006 and 91534109), National Key Projects for Fundamental Research and Development of China (No. 2017YFB0102200), Beijing Municipal Science and Technology Project (No. D171100005617001) and Henan province science and technology cooperation project (No. 172106000061).
| [1] |
S. Chu, A. Majumdar, Nature 488 (2012) 294-303. DOI:10.1038/nature11475 |
| [2] |
J.B. Goodenough, Energy Environ. Sci. 7 (2013) 14-18. |
| [3] |
J.B. Goodenough, Y. Kim, Chem. Mater. 2 (2010) 587-603. |
| [4] |
M. Zhu, Z. Wang, H. Li, et al., Energy Environ. Sci. 11 (2018) 941-951. DOI:10.1039/C7EE03232C |
| [5] |
J. Mao, K.H. Dai, M.J. Xuan, et al., ACS Appl. Mater. Interfaces 8 (2016) 9116-9124. DOI:10.1021/acsami.6b00877 |
| [6] |
L.H. Jiang, Q.S. Wang, J.H. Sun, J. Hazard. Mater. 351 (2018) 260-269. DOI:10.1016/j.jhazmat.2018.03.015 |
| [7] |
H. Li, C. Han, Y. Huang, et al., Energy Environ. Sci. 11 (2018) 2414-2422. DOI:10.1039/C8EE00590G |
| [8] |
J.J. Zhang, X. Zang, H.J. Wen, et al., J. Mater. Chem. A 5 (2017) 4940-4948. DOI:10.1039/C6TA10066J |
| [9] |
J. Mao, M.Z. Ma, P.P. Liu, et al., Solid State Ion. 292 (2016) 70-74. DOI:10.1016/j.ssi.2016.05.008 |
| [10] |
M. Herstedt, H. Rensmo, H. Siegbahn, et al., Electrochim. Acta 49 (2004) 2351-2359. DOI:10.1016/j.electacta.2004.01.016 |
| [11] |
R. Spotnitz, J. Franklin, J. Power Sources 113 (2003) 81-100. DOI:10.1016/S0378-7753(02)00488-3 |
| [12] |
S.B. Xia, F.S. Li, F.X. Chen, H. Guo, J. Alloys Compd. 731 (2017) 428-436. |
| [13] |
P.H. Huang, S.J. Chang, C.C. Li, J. Power Sources 338 (2017) 82-90. DOI:10.1016/j.jpowsour.2016.11.026 |
| [14] |
K. Liu, W. Liu, Y.C. Qiu, et al., Sci. Adv. 3 (2017) e1601978. DOI:10.1126/sciadv.1601978 |
| [15] |
G.Q. Ma, L. Wang, J.J. Zhang, et al., Prog. Chem. 28 (2016) 1299-1312. |
| [16] |
L.H. Jiang, Q.S. Wang, J.H. Sun, J. Hazard. Mater. 351 (2018) 260-269. DOI:10.1016/j.jhazmat.2018.03.015 |
| [17] |
K.C. Högström, H. Lundgren, S. Wilken, et al., J. Power Sources 256 (2014) 430-439. DOI:10.1016/j.jpowsour.2014.01.022 |
| [18] |
K. Kim, S. Ahn, H.S. Kim, et al., Electrochim. Acta 54 (2009) 2259-2265. DOI:10.1016/j.electacta.2008.10.043 |
| [19] |
H.P. Zhang, Q. Xia, B. Wang, et al., Electrochem. Commun. 11 (2009) 526-529. DOI:10.1016/j.elecom.2008.11.050 |
| [20] |
X.M. Zhu, X.Y. Jiang, X.P. Ai, et al., Electrochem. Acta 165 (2015) 67-71. DOI:10.1016/j.electacta.2015.02.247 |
| [21] |
C.A. de Wit, D. Herzke, K. Vorkamp, et al., Sci. Total Environ. 408 (2010) 2885-2918. DOI:10.1016/j.scitotenv.2009.08.037 |
| [22] |
L.S. Morf, J. Tremp, R. Gloor, et al., Environ. Sci. Technol. 39 (2005) 8691-8699. DOI:10.1021/es051170k |
| [23] |
S.H. Lee, C.S. Yoon, K. Amine, et al., J. Power Sources 234 (2013) 201-207. DOI:10.1016/j.jpowsour.2013.01.045 |
| [24] |
K. Xu, M.S. Ding, S.S. Zhang, et al., J. Electrochem. Soc. 149 (2002) A622-A626. DOI:10.1149/1.1467946 |
| [25] |
T. Subburaj, Y.N. Jo, C.W. Lee, Curr. Appl. Phys. 14 (2014) 1022-1027. DOI:10.1016/j.cap.2014.05.009 |
| [26] |
X. Wang, E. Yasukawa, S. Kasuya, J. Electrochem. Soc. 148 (2001) A1058-A1065. DOI:10.1149/1.1397773 |
| [27] |
R. Chen, Y. Zhao, Y. Li, et al., J. Mater. Chem. A 5 (2017) 5142-5147. DOI:10.1039/C6TA10210G |
| [28] |
K. Xu, Chem. Rev. 114 (2014) 11503-11618. DOI:10.1021/cr500003w |
| [29] |
Y. Huang, Y.C. Lin, D.M. Jenkins, et al., Acs Appl. Mater. Interfaces 8 (2016) 7013-7021. DOI:10.1021/acsami.5b12081 |
| [30] |
T. Yoon, M.S. Milien, B.S. Parimalam, B.L. Lucht, Chem. Mater. 29 (2017) 3237-3245. DOI:10.1021/acs.chemmater.7b00454 |
| [31] |
Y. Zhu, Y. Li, M. Bettge, D.P. Abraham, J. Electrochem. Soc. 159 (2012) 2109-2117. DOI:10.1149/2.083212jes |
| [32] |
Y.J. Lee, H.J. Kim, S.H. Do, J.Y. Kang, S.H. Lee, Sens. Actuators B Chem. 237 (2016) 924-934. DOI:10.1016/j.snb.2016.06.169 |
| [33] |
V.A. Sethuraman, L.J. Hardwick, V. Srinivasan, R. Kostecki, J. Power Sources 195 (2010) 3655-3660. DOI:10.1016/j.jpowsour.2009.12.034 |
| [34] |
Z.W. Lebens-Higgins, S. Sallis, N.V. Faenza, et al., Chem. Mater 30 (2018) 958-969. DOI:10.1021/acs.chemmater.7b04782 |
| [35] |
H.B. Xie, K. Du, G.R. Hu, et al., J. Mater. Chem. A 3 (2015) 20236-20243. DOI:10.1039/C5TA05266A |
| [36] |
A.R. Jimenez, R. Nolle, R. Wagner, et al., Nanoscale 10 (2018) 2128-2137. DOI:10.1039/C7NR06568J |
 2018, Vol. 29
2018, Vol. 29 

