b College of Mechanical and Electrical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
Novel high-energy-density energy storage systems are being pursued for the newly emerged applications, especially for electric vehicles and energy storage power stations. Recently, many efforts have been devoted to developing high capacity electrode materials to boost energy density of lithium-ion batteries (LIBs) [1-6]. With respect to anode materials, graphite has been widely used as anode materials in state-of-the-art LIBs due to its acceptable electrochemical performance and low cost. However, traditional graphite anodes can only offer a capacity of 372 mAh/g, which cannot meet the requirement of the aforementioned applications.
Silicon, one of the most promising anodes, has attracted great attention due to its high theoretical capacity of 4200 mAh/g in fully lithiated composition, Li4.4Si, which is ten times higher than that of commercial graphite [7-9]. Moreover, its earth abundance, low cost as well as the low discharge voltage further make it more attractive for the next generation LIBs. Despite lots of advantageous features, silicon anodes endure enormous volume expansion during lithiation/delithiation (~300%) [7, 10-12], which in turn leads to crush of silicon particles, collapse of electrode structure, and unstable solid-electrolyte-interphase (SEI) [13]. Consequently, the batteries undergo rapid capacity attenuation and low Coulombic efficiency.
To address the above issues, various strategies have been proposed including development of nanostructured silicon materials [14-18], novel electrolyte systems and additives [19-23]. Besides, exploiting functional binders to maintain the integrity and contribute to form a more stable SEI has been demonstrated as a simple and efficient way to mitigate the side reactions arising from large volume change of silicon. A serials of water soluble polymeric binders have been explored recently, such as carboxymethyl cellulose (CMC) [24], poly(acrylic acid) (PAA) [25], alginate [26] and hydrogen-bond-directed self-healing polymer [27]. The common ground of these polymers is that they can establish chemical bonding with silicon by their function groups. However, the polymer chains easily slip from silicon particles due to their liner structures [28]. Some researchers have developed crosslinked polymeric binders, such as PAA-PVA [28] and CMC-PAA [29]. The crosslinked polymer binder has a three-dimensional deformable network that can better accommodate large volume change of the silicon anode. Recently, the concept of mechanical bonds has been introduced to polymeric binder by integrating sliding-ring polyrotaxane to conventional polyacrylic acid binder, thus lowing stress originating from volume expansion of silicon [30]. Most of the current research use polymer binder system, nevertheless the study on the inorganic-organic polymer binder is lacking.
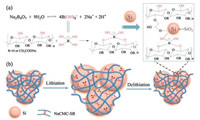
In this paper, a novel crosslinked hybrid binder is proposed by crosslinking sodium carboxymethyl cellulose (NaCMC) and sodium borate (SB), where the inorganic sodium borate was used as crosslinker. Coupled with three-dimensional network structure formed by crosslinking, the novel gel binder can effectively alleviate the volume expansion of the silicon anode as schematically shown in Fig. 1. The robust chemical bonds between silicon nanoparticles and gel binder lead to strong adhesion. Thus, this novel binder greatly improves cycle stability and cycle life of batteries. Considering the facile synthesis approach and good electrochemical performance, this hybrid binder can also be extended to other electrode materials going through substantial volumetric change.
|
Download:
|
| Fig. 1. (a) The chemical structure and illustrative interaction between sodium carboxymethyl cellulose, sodium borate and silicon nanoparticles; (b) Graphical representation of Si/NaCMC-SB configurations during lithiation/delithiation process. | |
The NaCMC-SB binder was synthesized through crosslinking sodium carboxymethyl cellulose by sodium borate. Typically, a mixture of aqueous NaCMC and sodium borate was heated at 85 ℃ to enable the hydrolysis of the sodium borate. It has been well demonstrated that sodium borate would readily hydrolyze into B(OH)4- as shown in Fig. 1a [31, 32]. This pretreated mixture was further directly used as binder for making silicon electrodes, followed by drying at 100 ℃ to allow the crosslinking via the condensation reaction between NaCMC and SB. The chemical structure and crosslinking reaction were schematically shown in Fig. 1a. It should be noted that the whole synthesis and electrode fabrication process were accomplished using water as solvent, avoiding the use of large amount of toxic and odor organic solvent, such as N, N-dimethylformamide (DMF) and dimethylacetamide (DMAC) in the Poly (vinylidene fluoride) (PVDF) binder. This ecofriendly, low-cost binder makes it more attractive for practical application.
The interaction between NaCMC, SB and silicon was investigated by FTIR and XPS. As shown in Fig. 2a, a strong broad peak centered at 3435 cm-1 was observed in the spectrum of NaCMC, which is attributed to the stretching vibration of the -OH group. This peak was found to downshift to a lower wavenumber of 3417 cm-1 for NaCMC-SB hybrid gel, which was mainly due to hydrogen bonds between -OH groups of NaCMC and borate ion of SB [33]. The NaCMC-SB sample displays new peaks at 1458 cm-1 and 1377 cm-1, which was assigned to the asymmetric peaks of B—O—C [32], indicating the crosslinking of NaCMC and SB. When applied as binder for silicon anode, the characteristic peak of —COO- at 1643 cm-1 for NaCMC-SB decreased to 1597 cm-1, suggesting that the —COO- groups of NaCMC could chemically bond with SiO2 thin layer of silicon nanoparticles [34]. Moreover, characteristic peak of —OH at 3417 cm-1 for NaCMC-SB shifted to 3398 cm-1 in the presence of Si, indicating that the occurrence of condensation reaction between —OH of sodium borate and Si—OH of Si. The chemical interaction between these components of the silicon electrode was schematically shown in Fig. 1a.

|
Download:
|
| Fig. 2. (a) The FT-IR spectra of NaCMC, SB and NaCMC-SB. (b) B 1s XPS spectra of NaCMC-SB and pure SB; (c) O 1s XPS spectra of NaCMC-SB and pure SB. | |
The chemical structure of these binders was further studied by X-ray photoelectron spectroscopy (XPS) shown in Figs. 2b and c. The peak centered at 192.5 eV for SB is assigned to B 1s in B—O bond, which is shifted to lower binding energy (191.95 eV) after crosslinking with NaCMC due to the formation of B—O—C bond [35]. In the O 1s spectra, the relatively broader peak at 531.5 eV for CMC-SB sample arises from B—O—C bond, which again verifies the occurrence of crosslinking [36]. The results from XPS analysis is highly consistent with FT-IR spectra.
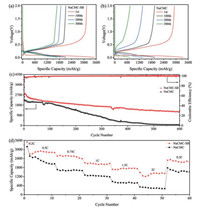
The electrochemical performance of the silicon anodes with NaCMC and NaCMC-SB hybrid gel binder was tested using a CR2016 coin cells with lithium foil as the counter electrode.1 mol/L LiPF6 in a mixture of ethylene carbonate/diethyl carbonate (EC/ DEC, 1:1 by volume ratio) with 10% fluoroethylene carbonate (FEC) as additive was used as electrolyte. The silicon electrodes with widely-used NaCMC binder in absence of any crosslinker was prepared as control sample. Typical galvanostatic voltage profiles of the silicon anodes with these two binder were shown in Fig. 3. Both these two samples had a characteristic discharge plateaus at 0.1 V for lithiation and a charge plateaus at 0.45 V for delithiation in the first cycle [8, 37]. The silicon anode with NaCMC-SB binder exhibited almost overlapped plateau with slight polarization increase even after 300 deep cycles shown in Fig. 3a. In contrast, large polarization was observed for the silicon anode with NaCMC binder upon the cycling.
|
Download:
|
| Fig. 3. Galvanostatic charge/discharge voltage profiles of the silicon anode using (a) NaCMC-SB and (b) NaCMC binder from 0.01-2 V. (c) The capacity and Coulombic efficiency of NaCMC-SB binder and NaCMC. (d) Rate capability of the Si anodes with NaCMC-SB and NaCMC binder (1C = 4000 mAh/g). | |
Fig. 3c shows the electrochemical performance of the Si anodes at 0.33 C (1C = 4000 mAh/g). The sample with NaCMC binder exhibited an initial capacity of 3353.2 mAh/g, whereas higher capacity (3524.2 mAh/g) was obtained for the NaCMC-SB gel binder. Additionally, NaCMC-SB binder delivered a higher initial Coulombic efficiency of 88.95% than that with NaCMC binder (86.73%) and rapidly rose to 97.89% within 3 cycles. The high efficiency is critical for real application in full cell that has limited lithium ion in the whole system. Noticeably, this gel binder makes the silicon anode maintain a high capacity of 1211.5 mAh/g after 600 cycles, whereas the control sample started descending quickly after 100 deep cycles and only 77.7 mAh/g remained after 600 cycles.
The rate capability of the silicon anode with NaCMC-SB hybrid binder and NaCMC were further evaluated with C-rate ranging from 0.2 C to 2 C. The sample with NaCMC-SB binder always displays higher capacity than that with NaCMC binder within the test range. In detail, the high capacity of ~3486.2 mAh/g, ~2890.5 mAh/g was achieved with NaCMC-SB binder at relatively low C-rate of 0.2 C and 0.5 C, respectively. The good rate capacity was demonstrated by the high capacity of 1468.9 mAh/g with increasing the current to 2 C. By contrast, NaCMC binder only left 490 mAh/g at 2 C. When the rate decreased back to 0.5 C, the NaCMC-SB/Si electrode delivered a discharge capacity of 2326.0 mAh/g practically returning to the capacity at preceding rate of 0.5 C, suggesting a good reversibility upon current change.
Surface morphology evolution of the silicon anodes with different binders was investigated by field emission scanning electron microscopy (FESEM), as shown in Fig. 4. The electrodes showed a similar morphology with silicon nanoparticles and carbon black uniformly dispersed in the matrix, indicating that both the NaCMC and NaCMC-SB had a good compatibility with the electrode components. However, obvious differences were observed between those two electrodes using NaCMC and NaCMC-SB binder after 50 deep cycles. The electrode with NaCMC binder exhibited significant cracks and voids and resulted in a loose structure. Whereas, the electrode components still connected with each other and no obvious crack were seen for the electrode with NaCMC-SB binder. The well-maintained electrode structure was believed due to the durable crosslinked network, which was also demonstrated by its much improved battery performance shown in Fig. 3. To better understand the reason for the improved cyclability and rate capability of silicon anode with hybrid binder, electrochemical impedance spectroscopy (EIS) was conducted on the cells before and after cycles as shown in Fig. S1 (Supporting information). The Nyquist plots of the Si electrodes after 1 and 10 cycles consist of two semicircles in the high-medium frequency region and a sloped line in the low frequency region. The first semicircle represents the contribution of the charge transfer between the electrolyte and SEI, while the other semicircle represents the charge transfer between the SEI and silicon. The charge transfer resistance (Rct) is reflected by the diameter of the first semicircle. The reduced Rct for the cell with NaCMC-SB binder confirms that more stable SEI is formed when using deformable hybrid binder. This observation is in line with the much improved battery performance of the silicon anode with NaCMC-SB gel binder.

|
Download:
|
| Fig. 4. Surface morphology of the silicon anodes (a) before and (b) after 50th cycle using NaCMC binder. The surface morphology of silicon anodes (c) before and (d) after 50th cycle using NaCMC-SB binder. | |
In conclusion, we have demonstrated a high capacity, long-cycle-life silicon anode based on a novel hybrid gel polymer binder through crosslinking sodium carboxymethyl cellulose by sodium borate acid. The gel polymer binder can effectively accommodate the large volume change upon cycling. With dual effect of crosslinking structure and chemical bonding between binder and silicon, the silicon anode displays extraordinary electrochemical properties with a high initial discharge capacity of 3524.2 mAh/g and high initial Coulombic efficiency of 88.95%. Moreover, the gel binder enables silicon anode achieve long-cycle-life (1211.5mAh/g after 600 deep cycles). Considering the good electrochemical performance and facile processing process, this developedhybrid gel polymer binder is quite attractive for those high capacity electrodes with large volume changes upon cycling.
AcknowledgmentsThis work is supported by the National Natural Science Foundation of China (No. 51602250) and Thousand Youth Talents Plan Project of China. We also would like to thank Mr. Ren and Miss Liu at Instrument Analysis Center of Xi'an Jiaotong University for their assistance with SEM and XPS analysis.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.03.008.
| [1] |
X. Zuo, J. Zhu, P. Mueller-Buschbaum, Y.J. Cheng, Nano Energy 31 (2017) 113-143. DOI:10.1016/j.nanoen.2016.11.013 |
| [2] |
X. Su, Q. Wu, J. Li, et al., Adv. Energy Mater. 4 (2014) 1300882. DOI:10.1002/aenm.201300882 |
| [3] |
J.R. Szczech, S. Jin, Energy Environ. Sci. 4 (2011) 56-72. DOI:10.1039/C0EE00281J |
| [4] |
M. Ashuri, Q. He, L.L. Shaw, Nanoscale 8 (2016) 74-103. DOI:10.1039/C5NR05116A |
| [5] |
L.Y. Liu, X. Zhang, H.X. Li, et al., Chin. Chem. Lett. 28 (2017) 206-212. DOI:10.1016/j.cclet.2016.07.027 |
| [6] |
Y. Chen, B. Wang, T. Hou, et al., Chin. Chem. Lett. 29 (2018) 187-190. DOI:10.1016/j.cclet.2017.06.019 |
| [7] |
X.H. Liu, H. Zheng, L. Zhong, et al., Nano Lett. 11 (2011) 3312-3318. DOI:10.1021/nl201684d |
| [8] |
B.A. Boukamp, G.C. Lesh, R.A. Huggins, J. Electrochem. Soc. 128 (1981) 725-729. DOI:10.1149/1.2127495 |
| [9] |
A.S. Arico, P. Bruce, B. Scrosati, J.M. Tarascon, W. Van Schalkwijk, Nat. Mater. 4 (2005) 366-377. DOI:10.1038/nmat1368 |
| [10] |
S.W. Lee, M.T. McDowell, J.W. Choi, Y. Cui, Nano Lett. 11 (2011) 3034-3039. DOI:10.1021/nl201787r |
| [11] |
X.H. Liu, L.Q. Zhang, L. Zhong, et al., Nano Lett. 11 (2011) 2251-2258. DOI:10.1021/nl200412p |
| [12] |
U. Kasavajjula, C. Wang, A.J. Appleby, J. Power Sources 163 (2007) 1003-1039. DOI:10.1016/j.jpowsour.2006.09.084 |
| [13] |
H. Wu, Y. Cui, Nano Today 7 (2012) 414-429. DOI:10.1016/j.nantod.2012.08.004 |
| [14] |
J. Ryu, D. Hong, S. Choi, S. Park, ACS Nano 10 (2016) 2843-2851. DOI:10.1021/acsnano.5b07977 |
| [15] |
X. Zhang, X. Qiu, D. Kong, et al., ACS Nano 11 (2017) 7476-7484. DOI:10.1021/acsnano.7b03942 |
| [16] |
Y. Jin, S. Li, A. Kushima, et al., Energy Environ. Sci. 10 (2017) 580-592. DOI:10.1039/C6EE02685K |
| [17] |
J. Lang, B. Ding, S. Zhang, et al., Adv. Mater. 29 (2017) 1701777. DOI:10.1002/adma.201701777 |
| [18] |
Z. Liu, X. Chang, T. Wang, et al., ACS Nano 11 (2017) 6065-6073. DOI:10.1021/acsnano.7b02021 |
| [19] |
G.T. Kim, T. Kennedy, M. Brandon, et al., ACS Nano 11 (2017) 5933-5943. DOI:10.1021/acsnano.7b01705 |
| [20] |
J. Zhao, L. Liao, F. Shi, et al., J. Am. Chem. Soc. 139 (2017) 11550-11558. DOI:10.1021/jacs.7b05251 |
| [21] |
Y. Horowitz, H.L. Han, P.N. Ross, G.A. Somorjai, J. Am. Chem. Soc. 138 (2016) 726-729. DOI:10.1021/jacs.5b10333 |
| [22] |
B. Philippe, R. Dedryvere, M. Gorgoi, et al., J. Am. Chem. Soc. 135 (2013) 9829-9842. DOI:10.1021/ja403082s |
| [23] |
C. Xu, F. Lindgren, B. Philippe, et al., Chem. Mater. 27 (2015) 2591-2599. DOI:10.1021/acs.chemmater.5b00339 |
| [24] |
N. Ding, J. Xu, Y. Yao, et al., J. Power Sources 192 (2009) 644-651. DOI:10.1016/j.jpowsour.2009.03.017 |
| [25] |
A. Magasinski, B. Zdyrko, I. Kovalenko, et al., ACS Appl. Mater. Interfaces 2 (2010) 3004-3010. DOI:10.1021/am100871y |
| [26] |
I. Kovalenko, B. Zdyrko, A. Magasinski, et al., Science 334 (2011) 75-79. DOI:10.1126/science.1209150 |
| [27] |
C. Wang, H. Wu, Z. Chen, et al., Nat. Chem. 5 (2013) 1042-1048. DOI:10.1038/nchem.1802 |
| [28] |
J. Song, M. Zhou, R. Yi, et al., Adv. Funct. Mater. 24 (2014) 5904-5910. DOI:10.1002/adfm.201401269 |
| [29] |
B. Koo, H. Kim, Y. Cho, et al., Angew. Chem. Int. Ed. 51 (2012) 8762-8767. DOI:10.1002/anie.v51.35 |
| [30] |
S. Choi, T.W. Kwon, A. Coskun, J.W. Choi, Science 357 (2017) 279-283. DOI:10.1126/science.aal4373 |
| [31] |
S.W. Sinton, Macromolecules 20 (1987) 2430-2441. DOI:10.1021/ma00176a018 |
| [32] |
B. Lu, F. Lin, X. Jiang, et al., ACS Sustain. Chem. Eng. 5 (2017) 948-956. DOI:10.1021/acssuschemeng.6b02279 |
| [33] |
U. Manna, S. Patil, J. Phys. Chem. B 113 (2009) 9137-9142. DOI:10.1021/jp9025333 |
| [34] |
Y. Liu, Z. Tai, T. Zhou, et al., Adv. Mater. (2017) 1703028. |
| [35] |
S.J. Wang, X.L. Xing, J. Li, X. Jing, J. Appl. Surf. Sci. 428 (2018) 912-923. DOI:10.1016/j.apsusc.2017.09.213 |
| [36] |
D.A. Hensley, S.H. Garofalini, Appl. Surf. Sci. 81 (1994) 331-339. DOI:10.1016/0169-4332(94)90290-9 |
| [37] |
T.D. Hatchard, J.R. Dahn, J. Electrochem. Soc. (2004) A838-A842. |
 2018, Vol. 29
2018, Vol. 29 

