Motivated by the rapid development of global economy, massive fossil consumption and increasing serious environmental pollution, it is ever urgent demands to seek other renewable and environmental friendly energy sources to reduce the dependence on fossil fuels which prompt the developments of storage and conversion technologies [1-4]. Additionally, the development of electric vehicles with lower pollution emissions and the high demanding of portable electronic devices, power tools and electric vehicles have accumulated tremendous research interests to probe high performance device to meet the requirements [5, 6].
Supercapacitors, also known as ultracapacitors or electrochemical capacitors, are famed for the higher power densities and longer cyclic lifespans than batteries, and higher energy densities than conventional electrostatic and electrolytic capacitors, stimulating world-wide attentions and explorations [7-9]. The energy storage of supercapacitors mainly depend on the contact surface of electrode materials with the electrolyte, different from the intercalation/deintercalation of Li+ within the bulk of electrode materials for lithium ion batteries which currently dominate the market of electronic devices. Furthermore, the supercapacitors deliver much higher power density than batteries, however, accompanying lower energy density than batteries, which can be seen from the Ragone plot in Fig. 1a.

|
Download:
|
| Fig. 1. (a) Ragone plots of different devices and the recent advanced reports of supercapacitors based on active materials. Reproduced with permission [4]. Copyright 2010, the Nature Publishing Group. (b) The number of recent reported literature associated with supercapacitors searching from Web of Science with the key words of "supercapacitors"at Nov. 01, 2018, and the inset is the cost of different energy storage systems (the data from ref [18]). | |
Though the supercapacitors possess a great deal of excellent properties, it is still a long way to be utilized in a large-scale commercialization [10]. The electrode materials, as one key component of supercapacitors, which are closely related to the energy density and power density, have induced considerable research interests to hunt high-performance supercapacitors [11]. Until now, there are large quantities of contributions about supercapacitors' electrode materials, which can be classified into three major categories (carbonaceous materials, conducting polymers, transition metal compounds) [12] such as graphene, activated carbon, carbon nanotubes, biomass, polyaniline (PANI), polypyrrole (PPy), MnO2, and RuO2, etc.
Until now, many ways have been explored to further enhance the performance of supercapacitors, such as enlarging the surface areas of materials, searching for new materials and introducing faradic redox reactions, etc. [4, 13, 14], which great promote the development of supercapacitors. Especially, some battery-type materials have been introduced and served as pseudocapacitors materials regardless the non-capacitor features [13, 15]. Typically, the nickel/cobalt based materials with lower price, abundant natural resources, environment-friendly and multiple oxidation states for richer redox reactions have received considerable research interests for supercapacitor electrode materials, such as nickel hydroxides and nickel cobaltite, etc. [16, 17]. Although some reviews have attempted to elucidate its unsuitability as a pseudocapacitor, there are still many works treated them as pseudocapacitor materials. Therefore, in this review, firstly we briefly summarize the fundamental mechanism of supercapacitors and classify them into three kinds according to the different energy storage mechanisms. Then we discuss the energy storage mechanism of nickel/cobalt based materials and suggest that these kinds of battery-type materials should be classified into hybrid supercapacitors instead of pseudocapacitors. Finally, we summerize the challenges of the nickel/cobalt based materials for commercialization in a large-scale.
2. Fundamental mechanism of supercapacitorsSupercapacitors not only store more energy (more than 80 Wh/kg, based on active materials) than conventional capacitors (usually less than 0.1 Wh/kg), but also exhibit economic power densities and energy densities (inset in Fig. 1b) [18]. Therefore, the supercapacitors have received tremendous research interests judging from the numbers of reports (Fig. 1b). However, there still exists conceptual confusion in the pursuit of high performance supercapacitors. In this section, we will state some definitions about the supercapacitors. Generally, supercapacitors can be divided into two major categories of non-faradic supercapacitors and faradic supercapacitors including EDLCs, pseudocapacitors (PCs) and hybrid supercapacitors (HSCs) according to their different charge storage mechanisms (Fig. 2a).
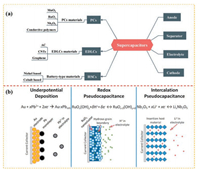
|
Download:
|
| Fig. 2. (a) The category of electrode materials and corresponding devices. (HSCs is hybrid supercapacitors and PCs is pseudocapacitors.) (b) Faradic redox reactions related to pseudocapacitive. Reproduced with permission [3]. Copyright 2013, Royal Society of Chemistry. | |
2.1. Non-faradaic supercapacitors
The EDLCs are developed from the electrical double-layer theory of Helmholtz model, which was later refined by Gouy, Chapman and Stern et al. [19-21] who took a diffuse layer in the electrolyte into account in order to more accurately describe the electrical double-layer theory. H. I. Becker first demonstrated and patented that the energy storage and delivery by EDLCs with porous carbon electrodes in an aqueous electrolyte in 1957 according to the electrical double-layer theory [22]. Then, NEC first brought the ELDCs-type devices into commercialization with the permission of SOHIO in 1978, which was first named as supercapacitors to describe the high energy differed from conventional capacitors [23].
For the EDLCs, the capacitance originates from charge accumulation at the electrode/electrolyte interface, where the charges are via pure physical adsorbing-desorbing charged ions from the electrolyte to the surface areas of the electrodes materials. It is obvious that the EDLCs are greatly similar to the parallel-plate capacitors [24, 25].
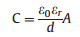
|
(1) |
where εr is the dielectric constant of electrolyte, ε0 is the permittivity of vacuum, d is the effective thickness of the electrical double layer, A is the effective area contacting with electrolyte.
There are no charges transfer between the interface of electrode materials and electrolyte which means non-faradic redox reactions are involved into this process. Therefore, the pure physical charge accumulation at the interfaces is responsible for high power density and long cycling life of the charge/discharge process with almost no volumes or morphologies changed for the electrode materials. Additionally, the capacitances of EDLCs are enormously affected by the effective thickness (d) and specific surface areas (A) as the Eq. (1) shows. The separation of electrodes and electrolytes for EDLCs is much smaller than that in conventional capacitors indicating that the EDLCs possess much higher specific capacitance [26].
Carbonaceous materials have received extensively and intensively consideration for the utilization of EDLCs, which are mainly due to their high specific surface areas, favourable electrical conductivity and abundant raw materials, etc. [27, 28]. Among various carbonaceous materials, the activated carbons are the first and most widely applied for supercapacitors, owing to their very high specific surface areas and their accessible raw materials (which can be easily obtained from petroleum by-products and biomass etc.) with low price. Clearly, the specific surface areas play a significant role in the value of specific capacitance. Hence, many approaches have been used to enlarge the specific surface areas, containing heating treatment under H2O steam or CO2 to remove partial carbon to produce pores, and employing activation agents of KOH, NaOH and ZnCl2 etc. to etch carbon to produce pores [29, 30]. The specific surface areas of activated carbons can be reached 3000 m2/g. However, the super-high specific surface areas have not brought high specific capacitance as expectation [25, 31]. These results demonstrate that not all the pores are involved into the contributions for specific capacitances, and the gravimetric capacitance is not all linearly increased with the specific surface areas. In fact, the gravimetric specific capacitances of activated carbons are linearly enlarged with their Brunauer-Emmet-Teller (BET) when the values are not too large [32]. Recent studies deliver that the specific capacitance can reach the maximum when the pore size is closed to the size of desolvated ions [33-35]. If the pore size is large enough to accommodate the diffusion layer, the area specific capacitance will tend to a certain value and the gravimetric specific capacitance will linearly change with the specific surface areas. Additionally, excessive pores will cause lower density and conductivity, which further affect the performance of supercapacitors. Therefore, many kinds of activated carbon are developed with the consideration of both the pore size distribution and volume/areas specific capacitance. Subsequently many other carbonaceous materials have been developed for supercapacitors, including carbon nanotubes, graphene and ordered porous carbon, etc. Furthermore, the performance of carbonaceous materials can be further enhanced by doping with heteroatoms (oxygen, nitrogen, sulfur and phosphorus etc.) and functionalization, with induce faradic redox reactions.
2.2. Faradaic supercapacitorsConway et al. first brought the reversible faradaic redox reactions into view, where the redox reactions occurring at or near the surface of electrode material displaying EDLC-like electrochemical features but the redox processes lead to much greater charge storage [4, 31]. However, to further improve the performaces, many efforts have been made to seek new materials and configurations. Although the electrochemical mechanism and behaviors of batteries are distinct from the supercapacitors, it is high feasibility to hybridize the supercapacitors and batteries due to their similar constructions in anodes, cathodes, electrolytes, separators and current collectors. Hence, many battery type materials have been introducing and serving as pseudocapacitors materials. Here, we will briefly summarize the fundamental mechanism of faradaic supercapacitors and classify them into two kinds according to the different energy storage mechanisms.
2.2.1. PseudocapacitorsDiffering from EDLCs, the pseudocapacitors rely on the fast reversible faradic redox reactions occurring at or near the surface of electrode materials exhibiting EDLCs-like electrochemical features. Compared with EDLCs, the performance of pseudocapacitors can be greatly enhanced by inducing faradic redox reactions on the surface and in the interior of electrodes materials (first few nanometres from the surface). Nevertheless, the faradic redox reactions also result in relatively lower power density and structural instability than EDLCs. What is more, it is worth noting that not all faradic redox reactions can be ascribed into pseudocapacitive behaviors as the batteries also are related to faradic reactions during charge/discharge. As shown in Fig. 2b, there are three kinds faradaic mechanisms can result in capacitive electrochemical features: underpotential deposition, redox pseudocapacitance, intercalation pseudocapacitance. Here, we summarize the general methods to distinguish the pseudocapacitive materials and battery-type materials.
Firstly, different from the thermodynamic behavior of batteries, the fast highly-reversible faradic redox reactions occurring at or near the surface of electrode materials lead to a EDLCs-like special potential-dependent charge accumulation or release phenomenon, in which the capacitance is determined by the derivative of dq/dV [36-38]. And these phenomenon results in EDLCs-like electrochemical behaviors (Figs. 3a and b) [39], where the CV curves are near rectangular and the GCD curves are isosceles triangular shape (Figs. 3c and d) [40]. By contrary, battery-type materials usually displayed apparent plateaus of the GCD curves during charge/discharge in virtue of the reactions occur at specific potential, and the CV curves exhibit clear peaks with broad peakto-peak voltage separation (Figs. 3e and f) [41].
Secondly, both the batteries and pseudocapacitors store energies by faradic reactions. Batteries usually involved phasetransformation during the process of charge/discharge, and the reaction is not confined to the interface of electrode materials and electrolyte. On the contrary, the faradic reactions for supercapacitors occur at the surface or near surface (first few nanometres from the surface), or are limited by the surface, are not limited by solid-state diffusion and therefore exhibit high rate capability. And there is no phase change involved into the reactions for supercapacitors.
Thirdly, the redox reactions of pseudocapacitive materials are not confined by the ion diffusion, but the battery-type materials are diffusion-controlled, for which the pseudocapacitor exhibit higher power density. It is can be determined by analyzing the peaks current and scant rates of CV curves according to the formula [42]:
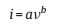
|
(2) |

|
(3) |
where i is the peak current and ν refer to the scan rates of CV curves, a, b are constant. For the capacitive materials, the value of b is 1 means that the peaks current is linear with the ν. And the value is 0.5 for diffusion-controlled battery-type materials. And the capacitive contribution content can be calculated according to the Eq. (3) for some electrode materials which the capacitances partly come from the pseudocapacitance and the value of b is close to 1. The k2v is the capacitive contribution, and k1v1/2 is the diffusioncontrolled capacity.
Fourthly, conducting polymers are promising pseudocapacitors materials in consideration of high conductivity and redox activity. The conductivity of the conductive polymer roots from that the carriers, under the electric field, move directionally along the longrange π-conjugated bond backbone. The redox reactions that offer the capacitance occurs through the ions adsorption-desorption on the backbone, which occur in the entire bulk materials, are not confined to the surface. There is no structural changes and phase changes during the redox reactions, therefore, the processes are highly reversible and pseudocapacitors behavior.
Additionally, differ from the redox pseudocapacitance, the faradic reactions of intercalation pseudocapacitance occur when cations (e.g., Li+, Na+, K+, and H+) intercalate/de-intercalate into the bulk of the active materials with no/or negligible crystallographic phase changes, where the redox reactions are not limited by the diffusion. There are broad peaks on the CV curves for intercalation pseudocapacitance, on which the peaks are shift indistinctly with the scan rates with a small peak-to-peak voltage separation (Fig. 3g). And the peaks currents of the CV curves for intercalation pseudocapacitance are linear with the scan rates (i = aνb, b = 1), and the GCD curves is almost linear under a wide potential window (Fig. 3h) [43].

|
Download:
|
| Fig. 3. The activated spherical microwave-expanded graphene oxide in [EMIM][TFSI]/AN electrolyte: (a) CV curves for different scan rates; (b) GCD curves under different current densities. Reproduced with permission [39]. Copyright 2013, American Chemical Society. MnO2: (c) GCD curves; (d) CV curves. Reproduced with permission [40]. Copyright 2018, ScienceDirect. (e) CV) curves of Fe3O4/G in the first three discharge-charge cycles at 0.5 mV/s; (f) Charge-discharge curves of Fe3O4/G electrode at different current densities of 0.1–3 C. Reproduced with permission [41]. Copyright 2013, Royal Society of Chemistry. (g) CV curves of H2Ti3O7 nanostructured electrode in 1 mol/L LiPF6 + EC/DMC (1/1, v/v) at various scan rates; (h) The linear relationship of the anodic and cathodic peak currents with the scan rates. Reproduced with permission [43]. Copyright 2005, American Chemical Society. | |
Remarkably, some battery-type materials deliver quasi capacitive behavior owing to the nanostructure shortens the diffusion paths when the size of materials approaches to nanoscale. This kind of materials are usually called extrinsic electrode materials, which is due to the high surface area decrease the diffusion distances and even suppress the phase transformation. Instead, the performance of intrinsic pseudocapacitive materials are not affected by the structures and morphologies, which display capacitive properties both in bulk materials and nanomaterials.
2.2.2. Hybrid supercapacitorsBesides the EDLCs and pseudocapacitors, another newly kind of supercapacitors is recently induced and named as hybrid supercapacitors, because the charge storage mechanisms and electrochemical behaviors are very different from the capacitive characteristics as the battery materials induced. Similar configuration of battery and supercapacitors make them feasible to combine into together. As expected, the hybrid supercapacitors integrate the high energy density of batteries as well as high power density and remarkable cycling life from supercapacitors, which impel them as the promising candidates for next generation energy storage devices. Although there are some reviews to distinct them with pseudocapacitors and entitled as hybrid supercapacitors [16], the confusion still exists especially for the nickel/cobalt based materials.
Here, we suggest that the hybrid supercapacitors (two electrodes devices) refer to the one whose electrodes adopt battery-type materials on the two-electrode devices, such as Ni(OH)2//AC hybrid supercapacitors. It must be noted that just only one electrode of the devices is non-capacitvie. Only in this case, these devices can be called hybrid supercapacitors. Otherwise, if both the two electrodes of devices are non-capacitive materials, we do not recommend these kinds devices to be named as hybrid supercapacitors, but rather as batteries.
Therefore, the hybrid supercapacitors exhibit higher energy density, but lower power densities than pseudocapacitors, which filed the gap of pseudocapacitors and batteries. Additionally, we propose that the symmetric and asymmetric supercapacitors should be correlated to the combination of capacitive materials, such as AC//AC symmetric supercapacitors and MnO2//AC asymmetric supercapacitors.
Since the battery-type materials exhibit non-capacitive properties with the CV possessing clear peaks with broad peak-to-peak voltage separation and GCD having obvious voltage plateau, the non-capacitive GCD and CV curves of battery-type materials, especially under three electrodes configuration, should be evaluated with the capacity instead of capacitance.
From the GCD curves:
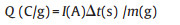
|
(4) |
or
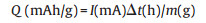
|
(5) |
where the Q (C/g or mAh/g) is the capacity, I is the constant current for the process, Δt is the discharge time, m refers to the mass/length/area/volume of active materials loading in the working electrode.
From the CV curves:
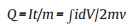
|
(6) |
where the Q (C/g or mAh/g) is the capacity, v is the scan rate, i is the instant current, ∫idV represents the areas of CV curves, and m refers to the mass/length/area/volume of active materials loading in the working electrode.
3. The mechanism of nickel/cobalt based materials for supercapacitorThe pseudocapacitors exhibit higher energy density than EDLCs and filled the gap between the EDLCs and batteries, triggering giant research interests. Although the RuO2-based materials for pseudocapacitors are considered as the promising candidates for next generation energy storage, the scarce and expensive resources of ruthenium greatly limit the commercialized in large-scale. Thus, the researchers turned their attention to other transition metal compounds, which possess multi-valence and high electrochemical activity for energy storage. And the nickel/cobalt based materials have attracted much attentions on account of their high electrochemical performance, environment-friendly and lower cost. However, the non-linear GCD curves and non-rectangular CV curves already demonstrate that this kind materials are noncapacitive materials. As the properties of nickel and cobalt materials are similar in many cases, the nickel and cobalt compound also can be obtained in any ratio. Herein, we put the nickel and cobalt materials together to discuss.
3.1. Nickel/cobalt hydroxides materialsNickel hydroxides are typical battery-type materials, which are widely used as positive electrode materials for Ni-MH batteries. With the prompt development of supercapacitors, nickel hydroxides have attracted considerable attentions for the supercapacitors electrode materials with their abundant resources, environmentally-friendly and high electrochemical activity. Yang et al. [44] have reported 3D porous Ni(OH)2 on nickel foam by electrodeposition with a maximum specific capacitance of 3152 F/g (about 1576 C/g or 437.8 mAh/g in actual) at 4 A/g, however, the capacitance only retained 280 F/g (140 C/g or 38.9 mAh/g) at 16 A/g and maintained 48% of the initial after 300 cycles. Wang et al. [45] synthesized porous honeycomb-like microstructure on nickel foam, which found that the structures, morphologies and performances were greatly affected by the electrodeposition temperature. And they obtained even higher capacitance with 3357 F/g (actually, 1678.5 C/g or 466.25 mAh/g) at 65 ℃. Ji et al. [46] synthesized nanoporous nickel hydroxide thin film on the surface of ultrathin-graphite foam, whose specific capacitance can reach (1.56 ± 0.06)×103 F/g (780 C/g or 216.7 mAh/g) at 0.5 A/g with the capacitance maintains approximately 65% after merely 1000 cycles at a current density of 10 A/g. It could be concluded that the nickel hydroxides suffered from much lower cycling performance and rate capability on account of the low electrical conductivity (only about 10-5~10-9 S/cm [47]) and poor structure stability during fast chargedischarge processes [48, 49]. In order to overcome this disturbing issue, various approaches have been involved to enhance the performance of Ni(OH)2. For instance, Wang et al. [50] grew Ni (OH)2 nanoplates on graphene exhibiting high specific capacitance of 1335 F/g (734.25 C/g or 204 mAh/g), favorable rate capability and excellent cycling stability (no obvious capacitance decrease after 2000 cycles at the high current density of 28.6 A/g). Ma et al. [51] have fabricated Ni(OH)2/graphene/bacterial cellulose paper hierarchical structured flexible electrode, which displayed high specific capacitance of 10.44 F/cm2 and 877.1 F/g (about 4.7 C/cm2 and 394.7 C/g) with a large mass loading of 11.9 mg/cm2 and excellent cycling stability with 93.6% capacitance retention after 15, 000 cycles. Besides, sponge-like Ni(OH)2 nanoparticles on MWCNTs [52], coaxial carbon nanotube/Ni (OH)2 composites [53] and hierarchical MnCo-layered double hydroxides@Ni(OH)2 core-shell heterostructures [54] etc. also extremely strengthen the electrochemical performances.
Remarkably, the conductivity and structure stability of Ni(OH)2 could also be improved with the introduction of cobalt. Compared to the solitary nickel/cobalt hydroxides, nickel-cobalt double hydroxides exhibited better electrochemical activity originating from the synergistic effect of nickel and cobalt which provide richer redox reactions, structure stability and electrical conductivity. Chen et al. [55] have prepared nickel-cobalt layered double hydroxide hybrid films on nickel foam delivered superior specific capacitance of 2682 F/g at 3 A/g (about 1341 C/g, 372.5 mAh/g) and the corresponding devices displayed higher energy density of 188 Wh/kg at 1499 W/kg. Warsi et al. [56] reported nickel-cobalt layered double hydroxides nanoflakes on fibrous carbon cloth, which displayed favorable specific capacitance of 1938 F/g (about 1162.8 C/g, 323 mAh/g) at 1 A/g, excellent rate capability with the capacitance retention of 79% at 50 A/g and satisfactory long-term endurance of 4.6% loss after 3000 cycles. Zha et al. [57] successfully synthesized acetate anion-intercalated nickel cobalt layered double hydroxides on Ni foam without additional alkali sources, which exhibited 2445 F/g (1222.5 C/g, 339.6 mAh/g) at 0.5 A/g and superior rate capability of 1383 F/g at 50 A/g, as well as remarkable cycling stability with 93% capacitance retention after 10, 000 cycles at 20 A/g.
However, Yury Gogotsi et al. have clearly pointed out that the Ni (OH)2 experience phase transformations and show sluggish kinetics with highly nonlinear charge/discharge and redox peaks in cyclic voltammograms should not be called supercapacitors [58]. The faradic redox reactions of nickel/cobalt hydroxides are expressed by the following accepted formulas [59, 60]:
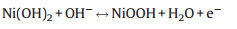
|
(7) |

|
(8) |
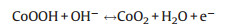
|
(9) |
It is obvious that the nickel/cobalt hydroxides were experience phase transformations from the above formulas. From the CV curves of nickel/cobalt hydroxides as depicted in Figs. 4a, d and g, it is easily obtained that the obvious redox peaks and nonrectangular characteristics revealing the non-capacitive properties. And the GCD curves (Figs. 4b, e and h) also exhibit nonlinearity and clear platform at about 0.25 V which are typical characteristics of battery-type materials as the faradic reactions occurs at a specific potential, further repealing the non-capacitive properties. Furthermore, the Figs. 4c, f and i show anodic peak current (ip) versus the square root of the scan rate (v1/2) obtained from the CV curves [61]. The linear properties of ip vs. v1/2 demonstrate that the redox reactions of Ni(OH)2 during the charge/discharge process are diffusion-controlled reactions corresponding to typical characteristics of battery-type materials. Remarkably, the GCD curves of nickel/cobalt hydroxides based materials for hybrid devices, in some cases, show extrinsic near linear characteristics thanks to the mutual complementarity of the positive and negative electrodes. Nevertheless, these characteristics do not change the nature of adopting battery-type materials as electrodes. Consequently, the nickel/cobalt hydroxides are typical battery-type materials, which should not be considered as pseudocapacitors materials. Here, we suggest that these kinds devices using nickel/cobalt hydroxides as one of the two electrodes should be called hybrid supercapacitors.
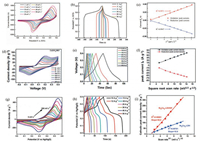
|
Download:
|
| Fig. 4. Ni(OH)2 on carbon fabric: (a) CV curves; (b) GCD curves; (c) the relationships of peak current with the square root of the scan rates rooting from CV curves. Co(OH)2 on multi-layered 3D graphene foam: (d) CV curves; (e) GCD curves; (f) the relationships of peak current with the square root of the scan rates rooting from CV curves. Reproduced with permission [61]. Copyright 2014, Royal Society of Chemistry. Nickel-cobalt layered double hydroxide on nickel foam: (g) CV curves; (h) GCD curves; (i) the relationships of peak current with the square root of the scan rates rooting from CV curves. Reproduced with permission [57]. Copyright 2017, ScienceDirect. | |
3.2. Nickel/cobalt oxides materials
The nickel oxide has been considered alternative electrode materials for supercapacitors because of its facile to synthesize various morphologies, environmentally benign nature and low costs [62, 63]. However, the NiO with wide band gap (3.6 eV [64]) has rather low electric conductivity which greatly affects the electrochemical performance including specific capacitance, rate capability and cycling stability. To address these issues, many smart and advanced electrodes have been utilized to promote the performance. Hierarchical porous NiO nanotube arrays displayed high capacitance of 675 F/g (270 C/g, 75 mAh/g) at 2 A/g, excellent rate capability with 569 F/g (227.6 C/g, 63.2 mAh/g) at high current density of 40 A/g, and outstanding life-span maintaining 93.2% after 10, 000 cycles [65]. Highly flexible NiO/MWCNTs nanohybrid thin films coating on stainless steel by the method of ionic layer adsorption and reaction exhibited high specific capacitance 1727 F/g (690.8 C/g, 191.9 mAh/g) at 5 mA/cm2 and 91% capacitance retention after 2000 cycles [66]. Recently, Wu et al. [67] have fabricated ultrathin porous NiO nanoflake arrays on nickel foam, in which the specific capacitance reached 2013.7 F/g (805.5 C/g, 223.7 mAh/g) at 1 A/g with remarkable cycling ability (negligible loss after 5000 cycles). And the as-assembled device of NiO//rGO delivers a high specific capacitance of 145 F/g and high energy density of 45.3 Wh/kg.
Despite the Co3O4 and NiO possess commendable properties, when one cobalt atom is replaced by nickel atom forming NiCo2O4 binarymetaloxides, the NiCo2O4 exhibit fascinating electrochemical performance. Compared with the NiO and Co3O4, NiCo2O4 based materials are particularly sought after, not only due to they possess almost all the merit of both NiO and Co3O4, but also they hold better electrical conductivity and structural stability. It has been reported that the electronic conductivity of single crystal NiCo2O4 nanoplate reached 62 S/cm and approximately 0.6 S/cm at 300 ℃ for polycrystalline NiCo2O4, which is at least two orders higher than solitary NiO (0.1 ×10-4 S/cm) and Co3O4 (0.8 × 10-4 S/cm) [68-70]. Therefore, there are so many advanced reports using many approaches with various morphologies and intriguing performances, such as 1D nanowires/microtubes, 2D nanosheets and 3D urchin-like structures/flower-shaped microspheres/hollow spheres etc. [71].
However, the nickel/cobalt oxides materials such as NiO, Co3O4 and NiCo2O4 etc. are typical battery-type materials and have been widely exploited as anode materials for lithium batteries, which should not be classified into pseudocapacitor materials. Both the apparent plateaus of the GCD curves during charge/discharge and clear peaks with broad peak-to-peak voltage separation of the CV curves illustrate the non-capacitive properties (Fig. 5) [72, 73]. Additionally, the ip against v1/2 are linear characteristics demonstrate that the redox reactions of NiCo2O4 during the charge/discharge process are corresponding to typical characteristics of battery-type materials. The extension lines of the linear fit for Figs. 5b, e and h are almost cross through the origin, which further state that the capacity is almost come from the non-capacitive contributions according to the Eq. 3. Therefore, the nickel/cobalt oxides possess the characteristics of typical battery-type materials, and should not be trated as pseudocapacitors materials.
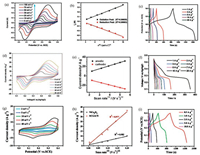
|
Download:
|
| Fig. 5. NiO on nickel foam: (a) CV curves; (b) the relationships of peak current with the square root of the scan rates rooting from CV curves; (c) GCD curves. Reproduced with permission [67]. Copyright 2016, Royal Society of Chemistry. mushroom-like Co3O4 hierarchitectures: (d) CV curves; (e) the relationships of peak current with the square root of the scan rates rooting from CV curves; (f) GCD curves. Reproduced with permission [72]. Copyright 2014, ScienceDirect. NiCo2O4 hexagonal nanoplates on reduced graphene oxide sheets: (g) CV curves; (h) the relationships of peak current with the square root of the scan rates rooting from CV curves; (i) GCD curves. Reproduced with permission [73]. Copyright 2015, ScienceDirect. | |
3.3. Other nickel and cobalt based materials
By virtue of the eminent electrochemical activity of nickel and cobalt based materials, there are still many other nickel and cobalt based materials with outstanding performance besides the materials as mentioned above. Guo et al. [74] synthesized NiMoO4 nanowires on carbon cloth and directly treated as high performance electrodes for supercapacitors, in which the specific capacitance achieved 1587 F/g (about 1032 C/g, 286.5 mAh/g) and displayed high energy density (70.7 Wh/kg) of corresponding device. Zhang et al. [75] reported NiMoO4@CoMoO4 hierarchical nanospheres and investigated the effect of Ni/Co molar ratios of raw materials on electrochemical behaviors. And they obtained that the specific capacitance were greatly enhanced by the advanced design and the synergistic effects of multi-component materials with high specific capacitance of 1601.6F/g (about 800.8 C/g, 222.4mAh/g) at the current density of 2A/g, as well as better cycling stability and rate capability. Interestingly, the NiMoO4@CoMoO4 hierarchical nanospheres exhibited higher specific capacitance of 1601.6F/g than the 1587F/g of NiMoO4 nanowires as mentioned above, however, the actual nanowires as mentioned above, however, the actual capacity was just opposite (1587F/g, 1032 C/g, 286.5mAh/g for NiMoO4@CoMoO4 hierarchical nanospheres, and 1601.6F/g, 800.8 C/g, 222.4mAh/g for the NiMoO4 nanowires) which further indicated the improper assessment of battery-type materials by using the capacitance.
Recently, nickel/cobalt based sulfide materials have also drawn sufficient considerations for the applications of supercapacitors electrodes materials in virtue of their excellent electronic conductivity and superior electrochemical activity. Especially, the nickel cobalt sulfides possess much higher electronic conductivity than the nickel cobalt oxides on account of these smaller band gap [76-78]. Nickel cobalt sulfide ball-in-ball hollow spheres have been reported by Shen et al. [78], which delivered a specific capacitance of 1036F/g (about 569.8C/g, 158.3mAh/g) at 1A/g and the as-assembled NiCo2S4//graphene/carbon spheres device displaying high energy density of 42.3Wh/kg at a power density of 476W/kg. Besides, interconnected nickel cobalt sulfide nanosheet arrays on carbon cloth were synthesized by one-step electrodeposition method [79], in which the specific capacitance reached 1418F/g (709C/g, 196.9mAh/g) at 5A/g and 1285F/g (642.5C/g, 178.5mAh/g) even at super-high current density of 100A/g demonstrating the excellent rate capability rooting from remarkable conductivity. And the corresponding Ni-Co-S//graphene device exhibited 60 Wh/kg at a power density of 1.8kW/kg, still maintained 33 Wh/kg at outstanding power density of 28.8kW/kg. Recently, Wang et al. [80] have prepared welldispersed nickel-cobalt sulfide nanoparticles on graphene delivered excellent electrochemical performances with the as-prepared Ni-Co-S@G//reduced graphene hydrogels device (RGH) exhibited 39.5 Wh/kg at 1778W/kg. Obviously, the non-rectangular CV, nontriangular GCD curves and the relationships of peak current with the squareroot of the scan rates as shownin Fig. 6 implied the noncapacitive performance about the aforementioned materials [81]. However, all these advanced reports of nickel/cobalt based materials expressed battery-type materials' characteristics and should not be relavant to the pseduocapacitors materials.

|
Download:
|
| Fig. 6. NiMoO4@CoMoO4 hybrid with Ni/Co molar ratio of 4:1: (a) CV curves; (b) the relationships of peak current with the square root of the scan rates rooting from CV curves; (c) GCD curves. Reproduced with permission [81]. Copyright 2015, Royal Society of Chemistry. 3D cauliflower-like NiCo2S4 architectures: (d) CV curves; (e) the relationships of peak current with the square root of the scan rates rooting from CV curves; (f) GCD curves. Reproduced with permission [76]. Copyright 2015, Royal Society of Chemistry. | |
Remarkably, almost all the specific capacity in alkaline electrolyte of nickel/cobalt based materials mainly originates from the faradaic redox reactions of Ni2+/Ni3+ and Co2+/Co3+/Co4+, which usually exhibit the characteristics of typical battery-type materials during the reactions. Therefore, the nickel/cobalt based materials in alkaline electrolyte should not associate with the pseudocapacitors, and the device who use these kinds of battery-type materials should be named as hybrid supercapacitors.
4. ConclusionsSupercapacitors bridge the gap of conventional capacitors and batteries, receiving substantial concerns and regarding as promising energy storage devices for next-generation. Since the electrode materials have crucial effect on the performance of integrate device, it is not exaggerative to pay enormous attentions on them. On account of the superior electrochemical properties, nickel/cobalt based materials have been widely exploited for supercapacitors electrode materials. In this review, we discussed the energy storage mechanism of nickel/cobalt based materials for supercapacitors. Then, we discussed the mechanism of nickel/cobalt based materials for supercapacitors. And owing to the noncapacitive and battery-type properties of nickel/cobalt based materials during the charge/discharge, we suggest that these kinds of materials should be classified into battery-type materials instead of pseudocapacitors materials, corresponding two-electrode devices should be named as hybrid supercapacitors. These hybrid supercapacitors with nickel/cobalt based materials will be filed the gap of pseudocapacitors and batteries (Fig. 7) [17, 82-91]. Additionally, despite the considerable advances in the nickel/cobalt based materials for supercapacitors, it still remains challenges.
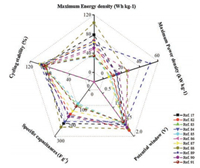
|
Download:
|
| Fig. 7. Some recent works about nickel/cobalt based materials. | |
Firstly, despite the nickel/cobalt based materials possess the properties of high electrochemical activities, low cost and environmental-friendly etc., the lower electrochemical potential windows (only approximately 0.5 V) are still the severely issue which confine them to satisfy the requirement in practical commercial applications. Combining with other negative electrode materials, especially the carbon materials, has been widely adopted to broaden the potential windows of the devices. However, the water decomposition of aqueous electrolyte cannot be ignored as the nickel/cobalt based materials have been treated favourable OER and HER electrode materials which will affect the security of the device, herein, it is better to evaluate the oxygen evolution overpotential of the nickel/cobalt based materials before assembled device.
Secondly, as the nickel/cobalt based materials are typical battery-type materials, phase changes are inevitable and the faradic redox reactions are bulk diffusion-controlled which not only lead to poor cycling stabilities but also result in lower rate capabilities greatly influencing on the power densities. We should not make the supercapacitors become batteries with high energy densities but low power densities. Therefore, we should design advanced electrodes and pursue both possess high energy density and power density.
Thirdly, the mass ratio of active materials should be more accessible to the commercial utilization, only in this case can the gravimetric capacitance more meaningful. And we should not assess the device in lower current densities and consider that these device possess high energy densities.
All in all, we strongly believe that the supercapacitors will have tremendous developments, and the nickel/cobalt based hybrid supercapacitors will be commercialized in large scale, which not only possesses supercapacitors' high power density but batteries' high energy density.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 51672109), Natural Science Foundation of Shandong Province for Excellent Young Scholars (No. ZR2016JL015).
| [1] |
P. Simon, Y. Gogotsi, B. Dunn, Science 343 (2014) 1210-1211. DOI:10.1126/science.1249625 |
| [2] |
C. Liao, Y. Wen, Z. Xia, et al., Adv. Energy Mater. 8 (2018) 1701621. DOI:10.1002/aenm.v8.9 |
| [3] |
V. Augustyn, P. Simon, B. Dunn, Energy Environ. Sci. 7 (2014) 1597-1614. DOI:10.1039/c3ee44164d |
| [4] |
P. Simon, Y. Gogotsi, Nat. Mater. (2010) 320-329. |
| [5] |
M.A. Hannan, M.M. Hoque, A. Mohamed, A. Ayo, Renew. Sust. Energy Rev. 69 (2017) 771-789. DOI:10.1016/j.rser.2016.11.171 |
| [6] |
J.R. Miller, P. Simon, Science 321 (2008) 651-652. DOI:10.1126/science.1158736 |
| [7] |
X. Wu, Z. Han, X. Zheng, et al., Nano Energy 31 (2017) 410-417. DOI:10.1016/j.nanoen.2016.11.035 |
| [8] |
W. Lv, R. Xue, S. Chen, M. Jiang, Chin. Chem. Lett. 29 (2018) 637-640. DOI:10.1016/j.cclet.2017.11.035 |
| [9] |
W. Jiang, F. Hu, Q. Yan, Wu Xiang, Inorg. Chem. Front. 4 (2017) 1642-1648. DOI:10.1039/C7QI00391A |
| [10] |
M.F. El-Kady, V. Strong, S. Dubin, R.B. Kaner, Science 335 (2012) 1326-1330. DOI:10.1126/science.1216744 |
| [11] |
Y. Shao, M.F. El-Kady, L.J. Wang, et al., Chem. Soc. Rev. 44 (2015) 3639-3665. DOI:10.1039/C4CS00316K |
| [12] |
J. Liu, J. Jiang, C. Cheng, et al., Adv. Mater. 23 (2011) 2076-2081. DOI:10.1002/adma.v23.18 |
| [13] |
Y. Wang, Y. Song, Y. Xia, Chem. Soc. Rev. 45 (2016) 5925-5950. DOI:10.1039/C5CS00580A |
| [14] |
W.J. Lu, S.Z. Huang, L. Miao, et al., Chin. Chem. Lett. 28 (2017) 1324-1329. DOI:10.1016/j.cclet.2017.04.007 |
| [15] |
T. Brousse, D. B'elanger, J.W. Long, J. Electrochem. Soc. 162 (2015) A5185-A5189. DOI:10.1149/2.0201505jes |
| [16] |
L.Y. Liu, X. Zhang, H.X. Li, et al., Chin. Chem. Lett. 28 (2017) 206-212. DOI:10.1016/j.cclet.2016.07.027 |
| [17] |
J. Yan, Z. Fan, W. Sun, et al., Adv. Funct. Mater. 22 (2012) 2632-2641. DOI:10.1002/adfm.201102839 |
| [18] |
B. Zakeri, S. Syri, Renew. Sust. Energ. Rev. 42 (2015) 569-596. DOI:10.1016/j.rser.2014.10.011 |
| [19] |
H. Helmholtz, Ann. Phys 165 (1853) 353-377. |
| [20] |
M. Gouy, J. Phys. Theor. Appl. 9 (1910) 457-468. DOI:10.1051/jphystap:019100090045700 |
| [21] |
O. Stern, Z. Elektrochem. Angew. Phys. Chem. 30 (1924) 508-516. |
| [22] |
H. I. Becker, Patent, US 2800616, 1957.
|
| [23] |
T. Pandolfo, V. Ruiz, S. Sivakkumar, J. Nerkar, Supercapacitors: Materials, Systems, and Applications, Wiley-VCH, 2013, pp. 69-109.
|
| [24] |
G. Wang, L. Zhang, J. Zhang, Chem. Soc. Rev. 41 (2012) 797-828. DOI:10.1039/C1CS15060J |
| [25] |
L.L. Zhang, X.S. Zhao, Chem. Soc. Rev. 38 (2009) 2520-2531. DOI:10.1039/b813846j |
| [26] |
X. Chen, R. Paul, L. Dai, Sci. Rev. 4 (2017) 453-489. |
| [27] |
Z.F. Tian, M.J. Xie, Y. Shen, Y.Z. Wang, X.F. Guo, Chin. Chem. Lett. 28 (2017) 863-867. DOI:10.1016/j.cclet.2016.12.004 |
| [28] |
M. Chen, Y. Yang, D. Chen, H. Wang, Chin. Chem. Lett. 29 (2018) 564-570. DOI:10.1016/j.cclet.2017.12.019 |
| [29] |
A. Ghosh, Y.H. Lee, ChemSusChem 5 (2012) 480-499. DOI:10.1002/cssc.v5.3 |
| [30] |
X. Li, Y. Chen, H. Huang, Y.W. Mai, L. Zhou, Energy Storage Mater. 5 (2016) 58-92. DOI:10.1016/j.ensm.2016.06.002 |
| [31] |
B.E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, Springer Science & Business Media, New York, 2013.
|
| [32] |
O. Barbieri, M. Hahn, A. Herzog, R. Kotz, Carbon 43 (2005) 1303-1310. DOI:10.1016/j.carbon.2005.01.001 |
| [33] |
J. Chmiola, G. Yushin, Y. Gogotsi, et al., Science 313 (2006) 1760-1763. DOI:10.1126/science.1132195 |
| [34] |
J. Chmiola, C. Largeot, P.L. Taberna, P. Simon, Y. Gogotsi, Angew. Chem. Int. Ed. 47 (2008) 3392-3395. |
| [35] |
D.T.L. Galhena, B.C. Bayer, S. Hofmann, G.A.J. Amaratunga, ACS Nano 10 (2015) 747-754. |
| [36] |
B.E. Conway, V. Birss, J. Wojtowicz, J. Power Sources 66 (1997) 1-14. DOI:10.1016/S0378-7753(96)02474-3 |
| [37] |
S. Zhang, N. Pan, Adv. Energy Mater. 5 (2015) 1401401. DOI:10.1002/aenm.201401401 |
| [38] |
B.E. Conway, W.G. Pell, J. Solid State Electrochem. 7 (2003) 637-644. DOI:10.1007/s10008-003-0395-7 |
| [39] |
T.Y. Kim, G. Jung, S. Yoo, K.S. Suh, R.S. Ruoff, ACS Nano 7 (2013) 6899-6905. DOI:10.1021/nn402077v |
| [40] |
W. Liu, M. Zhu, J. Liu, X. Lia, J. Liu, Chin. Chem. Lett. (2018). DOI:10.1016/j.cclet.2018.09.013 |
| [41] |
F. Zhang, T. Zhang, X. Yang, et al., Energy Environ. Sci. 6 (2013) 1623-1632. DOI:10.1039/c3ee40509e |
| [42] |
S. Ardizzone, G. Fregonara, S. Trasatti, Electrochim. Acta 35 (1990) 263-267. DOI:10.1016/0013-4686(90)85068-X |
| [43] |
J. Li, Z. Tang, Z. Zhang, Chem. Mater. 17 (2005) 5848-5855. DOI:10.1021/cm0516199 |
| [44] |
G.W. Yang, C.L. Xu, H.L. Li, Chem. Commun. 48 (2008) 6537-6539. |
| [45] |
Y.M. Wang, D.D. Zhao, Y.Q. Zhao, C.L. Xu, H.L. Li, RSC Adv. 2 (2012) 1074-1082. DOI:10.1039/C1RA00613D |
| [46] |
J. Ji, L.L. Zhang, H. Ji, et al., ACS Nano 7 (2013) 6237-6243. DOI:10.1021/nn4021955 |
| [47] |
C. Wang, E. Zhou, X. Deng, et al., Sci. Adv. Mater. 8 (2016) 1298-1304. DOI:10.1166/sam.2016.2725 |
| [48] |
S.I. Kim, S.W. Kim, K. Jung, J.B. Kim, J.H. Jang, Nano Energy 24 (2016) 17-24. DOI:10.1016/j.nanoen.2016.03.027 |
| [49] |
M. Yang, H. Cheng, Y. Gu, et al., Nano Res. 8 (2015) 2744-2754. DOI:10.1007/s12274-015-0781-3 |
| [50] |
H. Wang, H.S. Casalongue, Y. Liang, H. Dai, J. Am. Chem. Soc. 132 (2010) 7472-7477. DOI:10.1021/ja102267j |
| [51] |
L. Ma, R. Liu, L. Liu, et al., J. Power Sources 335 (2016) 76-83. DOI:10.1016/j.jpowsour.2016.10.006 |
| [52] |
D.P. Dubal, G.S. Gund, C.D. Lokhande, R. Holze, ACS Appl. Mater. Interfaces 5 (2013) 2446-2454. DOI:10.1021/am3026486 |
| [53] |
R.R. Salunkhe, J. Lin, V. Malgras, et al., Nano Energy 11 (2015) 211-218. DOI:10.1016/j.nanoen.2014.09.030 |
| [54] |
S. Liu, S.C. Lee, U. Patil, et al., J. Mater. Chem. A 5 (2017) 1043-1049. DOI:10.1039/C6TA07842G |
| [55] |
H. Chen, L.F. Hu, M. Chen, Y. Yan, L.-M. Wu, Adv. Funct. Mater. 24 (2014) 934-942. DOI:10.1002/adfm.v24.7 |
| [56] |
M.F. Warsi, I. Shakir, M. Shahid, et al., Electrochim. Acta 135 (2014) 513-518. DOI:10.1016/j.electacta.2014.05.020 |
| [57] |
D. Zha, H. Sun, Y. Fu, X. Ouyang, X. Wang, Electrochim. Acta 236 (2017) 18-27. DOI:10.1016/j.electacta.2017.03.108 |
| [58] |
Y. Gogotsi, ACS Nano (2014) 5369-5371. |
| [59] |
F. Lai, Y.E. Miao, L. Zuo, et al., Small 12 (2016) 3235-3244. DOI:10.1002/smll.v12.24 |
| [60] |
T. Wang, S. Zhang, X. Yan, et al., ACS Appl. Mater. Interfaces 9 (2017) 15510-15524. DOI:10.1021/acsami.7b02987 |
| [61] |
U.M. Patil, M.S. Nam, J.S. Sohn, et al., J. Mater. Chem. A 2 (2014) 19075-19083. DOI:10.1039/C4TA03953J |
| [62] |
F. Shi, L. Li, X. Wang, C. Gu, J. Tu, RSC Adv. 4 (2014) 41910-41921. DOI:10.1039/C4RA06136E |
| [63] |
J. Yan, Q. Wang, T. Wei, Z. Fan, Adv. Energy Mater. 4 (2014) 1300816. DOI:10.1002/aenm.201300816 |
| [64] |
J.M. Choi, S. Im, Appl. Surf. Sci. 244 (2005) 435-438. DOI:10.1016/j.apsusc.2004.09.152 |
| [65] |
F. Cao, G.X. Pan, X.H. Xia, P.S. Tang, H.F. Chen, J. Power Sources 264 (2014) 161-167. DOI:10.1016/j.jpowsour.2014.04.103 |
| [66] |
G.S. Gund, D.P. Dubal, S.S. Shinde, C.D. Lokhande, ACS Appl. Mater. Interfaces 6 (2014) 3176-3188. DOI:10.1021/am404422g |
| [67] |
S. Wu, K.S. Hui, K.N. Hui, K.H. Kim, J. Mater. Chem. A 4 (2016) 9113-9123. DOI:10.1039/C6TA02005D |
| [68] |
L. Hu, L. Wu, M. Liao, X. Hu, X. Fang, Adv. Funct. Mater. 22 (2012) 998-1004. DOI:10.1002/adfm.v22.5 |
| [69] |
Y. Fujishiro, K. Hamamoto, O. Shino, S. Katayama, M. Awano, J. Mater. Sci. 15 (2004) 769-773. |
| [70] |
A. Trunov, Electrochim. Acta 105 (2013) 506-513. DOI:10.1016/j.electacta.2013.05.028 |
| [71] |
C. Wang, E. Zhou, W. He, et al., Nanomaterials 7 (2017) 41. DOI:10.3390/nano7020041 |
| [72] |
F. Luo, J. Li, Y. Lei, et al., Electrochim. Acta 135 (2014) 495-502. DOI:10.1016/j.electacta.2014.04.075 |
| [73] |
L. Ma, X. Shen, H. Zhou, et al., Chem. Eng. J. 262 (2015) 980-988. DOI:10.1016/j.cej.2014.10.079 |
| [74] |
D. Guo, Y. Luo, X. Yu, Q. Lin, T. Wang, Nano Energy 8 (2014) 174-182. DOI:10.1016/j.nanoen.2014.06.002 |
| [75] |
Z. Zhang, Y. Liu, Z. Huang, et al., Phys. Chem. Chem. Phys. 17 (2015) 20795-20804. DOI:10.1039/C5CP03331D |
| [76] |
J. Pu, F. Cui, S. Chu, et al., ACS Sustainable Chem. Eng. 2 (2013) 809-815. |
| [77] |
S. Peng, L. Li, C. Li, et al., Chem. Commun. 49 (2013) 10178-10180. DOI:10.1039/c3cc46034g |
| [78] |
L. Shen, L. Yu, H.B. Wu, et al., Nat. Commun. 6 (2015) 6694. DOI:10.1038/ncomms7694 |
| [79] |
W. Chen, C. Xia, H.N. Alshareef, ACS Nano 8 (2014) 9531-9541. DOI:10.1021/nn503814y |
| [80] |
X. Wang, S. Zhao, L. Dong, et al., Energy Storage Mater. 6 (2017) 180-187. DOI:10.1016/j.ensm.2016.11.005 |
| [81] |
Y. Xiao, Y. Lei, B. Zheng, et al., RSC Adv. 5 (2015) 21604-21613. DOI:10.1039/C5RA00665A |
| [82] |
J. Huang, J. Wei, Y. Xiao, et al., ACS Nano 12 (2018) 3030-3041. DOI:10.1021/acsnano.8b00901 |
| [83] |
P. Sun, C. Wang, W. He, P. Hou, X. Xu, ACS Sustain. Chem. Eng. 5 (2017) 10139-10147. DOI:10.1021/acssuschemeng.7b02143 |
| [84] |
P. Sun, W. He, H. Yang, et al., Nanoscale 10 (2018) 19004-19013. DOI:10.1039/C8NR04919J |
| [85] |
C. Wang, K. Guo, W. He, et al., Sci. Bull. 62 (2017) 1122-1131. DOI:10.1016/j.scib.2017.08.014 |
| [86] |
J. Liao, P. Zou, S. Su, et al., J. Mater. Chem. A 6 (2018) 15284-15293. DOI:10.1039/C8TA04727H |
| [87] |
J.S. Wei, H. Ding, P. Zhang, et al., Small 12 (2016) 5927-5934. DOI:10.1002/smll.201602164 |
| [88] |
J. Zhao, Z. Li, X. Yuan, et al., Adv. Energy Mater. 8 (2018) 1702787. DOI:10.1002/aenm.v8.12 |
| [89] |
J. Yang, C. Yu, X. Fan, J. Qiu, Adv. Energy Mater. 4 (2014) 1400761. DOI:10.1002/aenm.201400761 |
| [90] |
W. He, C. Wang, H. Li, et al., Adv. Energy Mater. 7 (2017) 1700983. DOI:10.1002/aenm.201700983 |
| [91] |
W. He, G. Zhao, P. Sun, et al., Nano Energy 56 (2019) 207-215. DOI:10.1016/j.nanoen.2018.11.048 |
 2018, Vol. 29
2018, Vol. 29 

