Owning to efficiency, versatility and convenience, visible lightdriven photocatalytic decolorization is of great importance for treatment of pollutes containing synthetic dyes [1-3]. As a novel class of carbon-based nanomaterials, graphene quantum dots (GQDs) are defined as graphene sheets with few to ten layers and lateral dimensions < 100 nm [4, 5]. Zero-dimensional (0D) GQDs exhibit outstanding optical activity, robust chemical inertness due to their quantum confinement and edge effects [6-9]. Such superior physicochemical properties along with their low-toxicity and excellent biocompatibility endow them with potential applications in various fields, such as sensing, photocatalysis and bioimaging [4-13]. In recent researches, GQDs were applied as sensitizers to form composites with traditional photocatalytic materials (e.g., mesoporous graphite carbon nitride, TiO2, titania, etc.) for improved performance due to the ability to extremely facilitate the charge migration and prolong the charge lifetimes by suppressing the recombination of photogenerated electrons and holes [14-16]. However, GQDs usually lack broad absorption in the visible region and emission under long wavelength excitation. Therefore, controlling the properties of GQDs and exploring their application as green nanomaterials for the visible-light-driven photocatalysis are of great significance.
Recently, doping GQDs with heteroatoms has provided an attractive way to effectively change their electronic density and tune intrinsic properties [17]. Moreover, the photoexcited GQDs are excellent electron acceptors and donors [18], which can act as efficient visible light induced nanophotocatalyst. Nowadays, several literatures have been reported for the successful doping of GQDs with heteroatoms for ion detection, including N-doped and S, N co-doped GQDs [12, 19]. Nevertheless, as we known, the element doped GQDs have not been employed in the visible lightdriven photocatalytic application.
Herein, we developed a facile hydrothermal strategy to prepare S-GQDs using 1, 3, 6-trinitropyrene and Na2S as carbon and sulfur precursors. Compared to traditional semiconductor QDs, the asobtained S-GQDs are chemical inertness that could be used as a safe and green photocatalyst for fast degradation of basic fuchsin (BF) with excellent performance.
1, 3, 6-Trinitropyrene was synthesized according to the literature [20]. 20.0 mg of 1, 3, 6-trinitropyrene were ultrasonically dissolved in NaOH (0.15 mol/L) solution containing Na2S (0.1 mol/L), and the mixture was then hydrothermally treated at 180 ℃ for 4 h. The relatively uniform size of S-GQDs was obtained through dialysis of the solution (with cut-off molecular weight of 1, 000 Da). The control GQDs without S dopping was also prepared when the trinary source materials for S-GQDs were changed to binary 1, 3, 6-trinitropyrene and NaOH under otherwise same conditions. The product was named as S-free GQDs as for comparison.
Transmission electron microscope (TEM) measurement was made on a JEM-2100 TEM with an accelerating voltage of 200kV. Atomic forcemicroscopic (AFM) imagewas obtained byNanascopy IVA system. Elemental analysis of the S-GQDs was performed by Xray photoelectron spectroscopy (XPS) with PHI5300 electron spectrometer. UV-vis absorption and fluorescence spectra were recorded on a UV-2450 spectrophotometer and an RF-5301PC spectrofluorometer. Powder X-ray diffraction (XRD) patterns of samples were collected on a DX-2700 diffractometer (Dandong Haoyuan Instrument Co., Ltd.) using Cu Kα radiation. Zeta potential was recorded on Surpass zeta potential system (Anton parr, Austria).
1.0mL of catalyst (7.5mg/mL) was added into 50.0mL of BF solution (5.0mg/L) in a reactor at room temperature. Before illumination, the suspension was stirred for 1h in dark to ensure adsorption equilibrium. A 300W xenon lamp with a cutoff filter (λ > 420m) was used as the light source. About 3.5mL of suspension was taken out every 20min, and the BF in the suspension was quantified by measuring the absorbance at λmax=542nm. Degradation efficiency (D%) was calculated as (C0 - Ct)/C0 ×100%, in which C0 and Ct were initial concentrations of BF solution at the time of 0 and t, respectively. Unlike bulk powder materials, S-GQDs are soluble (well-dispersed) in water and no phase separation of dye occurred. Thus, the decrease of BF concentration caused by adsorption could be ignored. Thus, C0 is 5.0mg/L. All incubation time in the degradation process is 120 min.
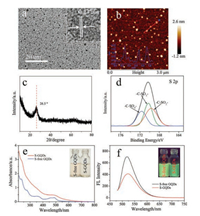
As shown in TEM image (Fig. 1a), the well-dispersed S-GQDs have a relatively narrow size distribution between 2.0 ~4.5nm (Fig. S1 in Supporting information). From the high-resolution TEM (HRTEM) image (inset of Fig. 1a), a clear lattice parameter is 0.23nm that is attributed to the (1120) facet of graphene [21]. This result indicated the S-GQDs with good crystallinity are almost perfect single crystals. AFM image in Fig. 1b presents the topographic height of S-GQDs around 1.4nm, suggesting that most of the fabricated S-GQDs consist of two graphene layers. The S-GQDs exhibits a remark (002) signal centered at around 26.5° (Fig. 1c) corresponding to the typical XRD pattern of the pristine graphite [22]. The XPS survey spectrum of the S-GQDs (Fig. S2a in Supporting information) displays three peaks at 534, 287 and 151eV, which respectively correspond to O 1s, C 1s and S 2p. The content of S atoms is 1.69%. The result confirms the successful Sdoping via the hydrothermal way. The S 2p spectrum of S-GQDs (Fig. 1d) can be divided into three apparent peaks at 169.2, 168.4 and 167.4eV, corresponding to the -C-S(Ox)-C- (x=2, 3, 4) sulphone bridges [23]. Thus, S atoms are covalently bonded to the framework of GQDs. The C1s spectrum of S-GQDs (Fig. S2b in Supporting information) could be assigned to C-O, C=C and C-S, respectively. The O 1s spectrum of S-GQDs (Fig. S2c in Supporting information) indicates C-OH or C-O-C. A clear blue-shift in the UV-vis adsorption spectra (Fig. 1e) is observed for S-GQDs in comparison with S-free GQDs, indicating the S dopant distinctly changes the electronic structure of GQDs. According to the onset of adsorption, the band gap of S-GQDs is estimated to be 2.53eV. Furthermore, the FL of S-GQDs and S-free GQDs are studied in Fig. 1f, where S-free GQDs exhibits stronger emission intensity than S-GQDs under the same excitation wavelength (460nm). The weakened FL emission implies that the charge recombination is inhibited in S-GQDs.
|
Download:
|
| Fig. 1. (a) TEM image (HRTEM image as inset), (b) AFM image (the inset is the height profile of marked line), (c) XRD and (d) high-resolution S 2p spectrum of S-GQDs. (e) UV- vis absorption spectra and (f) fluorescence (FL) spectra of S-GQDs and S-free GQDs. | |
The experimental parameters for photo-degradation of BF including pH value, dye concentration and dosage of S-GQDs were optimized. As revealed by zeta potential, S-GQDs exhibits negative charge (Fig. S3 in Supporting information) due to the existence of -OH and sulphone structure. Thus the degradation ratio increases as enhancing the solution pH because of the competitive adsorption of H+ with dye cations on S-GQDs at low pH values (Fig. 2a). The D% reaches the maximum value at the pH of 7.0. As the structure of BF changes at alkaline condition by turning large conjugate structure in BF into non-conjugated three-benzene ring structure [24], the D% decreases at pH 8.0 (Fig. S4 in Supporting information). Thus, we chose 7.0 as the optimal pH range. The degradation of BF (5.0 mg L) at different amount of catalysts was investigated in Fig. 2b. It is clearly found that 1.0 mL of S-GQDs is the optimum catalyst dosage. Also, the effect of initial BF concentration on degradation efficiency was studied in Fig. 2c. As shown the degradation ratio firstly increased at the low concentration and then decreased at the high concentration. That might be ascribed to the dye sensitization effect. At low concentration, few BF molecules can be adsorbed on the GQD surface, and the photo-induced charges are mainly produced from the excition of GQDs. with the increase of BF concentration, the adsorption became significant. Under the visible light illumination, lots of photo-excited electrons on the adsrobed BF molecules transfered to GQDs. This resulted in the enhancement of degradation ratio from 1 mg/L to 4 mg/L. However, as the concentration continued to be improved, the free BF molecules increased, causing the decrease of degradation ratio.

|
Download:
|
| Fig. 2. The effect of pH value (a), dosage of S-GQDs (b) and initial dye concentration (c) on D%. (d) The change of BF absorption spectra with S-GQDs under visible light (the comparative images of BF solution before and after irradiation as inset). (e) The comparison of D% and (f) the corresponding pseudo-first-order kinetics fitting under various experimental conditions. | |
Fig. 2d depicts the spectral change of BF solution under the optimized experimental conditions. The absorption peak (542 nm) decreases gradually under visible light, and the solution becomes almost colorless after 2 h illumination as illustrated in the inset of Fig. 2d. Fig. 2e compares the removal efficiency at various experimental conditions. BF is an extremely stable dye under visible light, and the photolysis ratio of BF (5.0 mg/L) is 4% after 2 h. Addition, the superior stability of S-GQDs in the BF solution is demonstrated by the adsorption test in dark, where the unchanged BF concentration suggests the addition of S-GQDs does not result in the flocculation. We also compared the activity of S-free GQDs that merely photo-degrade 18% BF in 2 h. Nevertheless, the S-GQDs can efficiently remove 81% dye after 2 h illumination. The weak photodegradation efficiency of S-free GQDs might be ascribed the relatively low absorbance of visible light (Fig. 1e) and the weak matching of energy level with BF. For well understanding the photocatalytic process, these experimental data are fitted by a pseudo-first-order kinetic equation: ln(C0/Ct) = kt (where k is the apparent rate constant), as shown in Fig. 2f. The obtained k value for S-GQDs is 0.0137 which is 8.6 times higher than that of S-free GQDs. The stability of S in S-GQDs after dye degradation process is investigated by XPS. The solution after dye degradation process is treated using ultrafiltration (with cut-off molecular weight of 1000 Da) to recover the S-GQDs. S content is 1.58% that is close to the initial value, indicating stability of S atoms in S-GQDs. The stability could be ascribed to the covalent bond of S atoms to skeleton of GQDs
In general, three main active species are involved in the photocatalytic process, holes (h+), hydroxyl radical (·OH) and superoxide radical (·O2-). The ·O2- generation is examined by the degradation of nitrotetrazolium blue (NBT) [25]. As shown in Fig. 3a, the UV-vis absorption of NBT gradually decreases, demonstrating the production of ·O2- radicals. Also, the detection of ·OH radicals [26, 27] is studied by measuring FL spectra of generated 2-hydroxy terephthalic acid (TAOH) at excitation wavelength 320 nm in Fig. 3b. The gradual improvement of emission intensity reveals the generation of ·OH radicals under visible light. Moreover, the main active species are determined by the trapping experiments with various scavengers (Fig. 3c), such as para-benzoquinone (BQ) as ·O2- radical scavenger, tertiary butanol (TBA) as ·OH radical scavenger and disodium ethylenediaminetetraacetate (EDTA-2Na) as holes scavenger. It can be obviously found that the photocatalytic efficiency is markedly prohibited after adding the above three scavengers, implying that all of these three species attend the photocatalysis reaction. Based on the above discussion, the corresponding photocatalytic mechanism of S-GQDs under visible light irradiation is shown in Fig. 3d. As illustrated, under the visible light irradiation, the electrons on the HOMO level of S-GQDs are excited and transitedto the LUMO level, meanwhile leaving photoinduced holes on the HOMO level. The photoexcited electrons on the LUMO level of SGQDs subsequently reduces the adsorbed O2 to the ·O2- radicals that could be further transformed to another oxidizing radicals of ·OH radicals. Finally, BF dyes are decomposed by these three kind of active species, h+, ·O2- and ·OH.

|
Download:
|
| Fig. 3. (a) Photocatalytic molecular oxygen activation activity for ·O2- generation by S-GQDs in NBT solution. (b) FL spectra of TAOH from the oxidation of terephthalic acid (TA)bygenerated ·OH in S-GQDs. (c) Photocatalytic activity of S-GQDs nanophotocatalyst with differentscavenges.(d) The possible mechanism of photocatalytic removing BF by the S-GQDs. | |
In summary, we have developed a facile hydrothermal synthesis of S-GQDs with uniform size and good crystallinity. These S-GQDs have a broad adsorption in visible region and a high separation ratio of photogenerated charges, which results in an enhanced photocatalytic performance for removing BF dyes under visible light irradiation in comparison with S-free GQDs. The improved photocatalytic activity from the successful doping of S element provides a strategy to tune the property of GQDs.
AcknowledgmentsThe authors gratefully acknowledge the financial support from the Zhejiang Provincial Natural Science Foundation of China (Nos. LY17B050007, LY15B050006) and 521 Talent Project of ZSTU.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.004.
| [1] |
Y. Guo, M.W. Huang, X.L. Fu, et al., Chin. Chem. Lett. 28 (2017) 719-728. DOI:10.1016/j.cclet.2017.02.006 |
| [2] |
H.X. Wang, R. Wu, S.H. Wei, et al., Chin. Chem. Lett. 27 (2016) 1572-1576. DOI:10.1016/j.cclet.2016.03.003 |
| [3] |
H.Y. Hao, Y.Y. Xu, P. Liu, G.Y. Zhang, Chin. Chem. Lett. 26 (2015) 133-136. DOI:10.1016/j.cclet.2014.11.022 |
| [4] |
S.N. Baker, G.A. Baker, Angew. Chem. Int. Ed. 49 (2010) 6726-6744. DOI:10.1002/anie.200906623 |
| [5] |
L.A. Ponomarenko, F. Schedin, M.I. Katsnelson, et al., Science 320 (2008) 356-358. DOI:10.1126/science.1154663 |
| [6] |
X. Li, M. Rui, J. Song, Z. Shen, H. Zeng, Adv. Funct. Mater. 25 (2015) 4929-4947. DOI:10.1002/adfm.v25.31 |
| [7] |
X.T. Zheng, A. Ananthanarayanan, K.Q. Luo, P. Chen, Small 11 (2015) 1620-1636. DOI:10.1002/smll.v11.14 |
| [8] |
J. Ju, W. Chen, Anal. Chem. 87 (2015) 1903-1910. DOI:10.1021/ac5041555 |
| [9] |
N. Li, A. Than, C.C. Sun, et al., ACS Nano 10 (2016) 11475-11482. DOI:10.1021/acsnano.6b07237 |
| [10] |
X. Sun, Y. Qian, Y. Jiao, et al., Talanta 165 (2017) 429-435. DOI:10.1016/j.talanta.2016.12.085 |
| [11] |
S. Bian, C. Shen, Y. Qian, et al., Sens. Actuators B-Chem. 242 (2017) 231-237. DOI:10.1016/j.snb.2016.11.044 |
| [12] |
S. Bian, C. Shen, H. Hua, et al., RSC Adv. 74 (2016) 69977-69983. |
| [13] |
M. Roushani, M. Mavaei, H.R. Rajabi, J. Mol. Catal. A-Chem. 409 (2015) 102-109. DOI:10.1016/j.molcata.2015.08.011 |
| [14] |
J. Liu, H. Xu, Y. Xu, et al., Appl. Catal. B-Environ. 207 (2017) 429-437. DOI:10.1016/j.apcatb.2017.01.071 |
| [15] |
H. Xie, C. Hou, H. Wang, Q. Zhang, Y. Li, Nanoscale Res. Lett. 12 (2017) 400. DOI:10.1186/s11671-017-2101-1 |
| [16] |
S. Bian, C. Zhou, P. Li, et al., ChemCatChem 9 (2017) 3349-3357. DOI:10.1002/cctc.201601594 |
| [17] |
Y. Du, S. Guo, Nanoscale 8 (2016) 2532-2543. DOI:10.1039/C5NR07579C |
| [18] |
X. Wang, L. Cao, F. Lu, et al., Chem. Commun. 25 (2009) 3774-3776. |
| [19] |
J. Ju, W. Chen, Biosens. Bioelectron. 58 (2014) 219-225. DOI:10.1016/j.bios.2014.02.061 |
| [20] |
L. Wang, Y. Wang, T. Xu, et al., Nat. Commun. 5 (2014) 5357. DOI:10.1038/ncomms6357 |
| [21] |
D. Qu, M. Zheng, P. Du, et al., Nanoscale 5 (2013) 12272-12277. DOI:10.1039/c3nr04402e |
| [22] |
M. Zhang, L. Bai, W. Shang, et al., J. Mater. Chem. 22 (2012) 7461-7467. DOI:10.1039/c2jm16835a |
| [23] |
S. Li, Y. Li, J. Cao, et al., Anal. Chem. 86 (2014) 10201-10207. DOI:10.1021/ac503183y |
| [24] |
R.J. Lan, J.T. Li, B.H. Chen, J. Photoenergy 2013 (2013) 893131. |
| [25] |
X. Jin, L. Ye, H. Wang, et al., Appl. Catal. B-Environ. 165 (2015) 668-675. DOI:10.1016/j.apcatb.2014.10.075 |
| [26] |
R. Marschall, A. Mukherji, A. Tanksale, et al., J. Mater. Chem. 21 (2011) 8871-8879. DOI:10.1039/c0jm02549f |
| [27] |
J. Fu, Y. Tian, B. Chang, F. Xi, X. Dong, J. Mater. Chem. 22 (2012) 21159-21166. DOI:10.1039/c2jm34778d |
 2018, Vol. 29
2018, Vol. 29 


