b Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, Wuhan 430074, China;
c Technical Center of Fujian Tobacco Industrial Co., Ltd., Xiamen 361022, China
Hydrogen has been regard as the most promising energy carrier for future sustainable energy conversion and storage [1-8]. However, the search for safe and efficient storage of hydrogen still remains the most challenging barriers for the upcoming "hydrogen economy" [9-11]. To date, a large number of hydrogen storage approaches have been developed [12-23], among which ammonia borane (NH3BH3, AB) has received intensive interest, owning to its high stability, high hydrogen content (19.6 wt%), and environmental benignity [24-29]. As shown in Eq. (1), a unit of AB is able to release three units of hydrogen though the catalytic hydrolysis process [30].
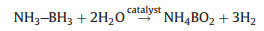
|
(1) |
By far, precious Ru-/Rh-based and Pt-based nanocomposites are considered as the most efficient catalysts for catalytic hydrolysis of ammonia borane [31-33]. However, the high cost and low abundance greatly limit their further practical applications. Recently, Co-based noble-metal-free catalysts have been widely studied for catalytic hydrolysis of AB. For instance, Hong et al. reported the synthesis of amine-capped Co nanoparticles by in situ deposition method and their catalytic activity toward hydrolysis of ammonia borane with the turnover frequency (TOF) value of 39.9 min-1 [34]. Recently, our group reported the synthesis of graphene supported cobalt (0) and CoNi nanoparticles via in situ one-step procedure, and their catalytic performance toward hydrolysis of ammonia borane with the turnover frequency (TOF) values of 13.8 min-1 and 16.4 min-1, respectively [35, 36]. Despite great efforts have been made, the catalytic activities toward hydrolysis of AB are still unsatisfactory. Therefore, it is highly desirable to develop the noble-metal-free catalysts with high activity and stability, which is crucial for promoting the potential application of ammonia borane as a hydrogen storage material, but still remains big challenging [37].
On the other hand, it has been reported that the introduction of cerium could increase the charge transfer and energy conversion efficiency of the metal centers, resulting in the enhancement of catalytic performance [38, 39]. For example, Yong et al. reported the synthesis of CuNi-CeO2/rGO hybrid as an efficient catalyst for hydrolysis of ammonia borane with a TOF value of 34.4 min-1, which is much higher than that without ceria [40]. Wang and coworkers reported Ni-Pt bimetallic nanocomposites anchored on mesoporous ceria with a TOF of 293 h-1 for complete dehydrogenation of hydrous hydrazine at 303 K, which is far better than that without ceria [41].
Following this strategy, herein, we reported a successful synthesis of the Co-CeOx nanoclusters anchored on nitrogendoped graphene hydrogel (Co-CeOx/NGH). The NGH was chosen as the carrier substrate for arching the as-synthesized Co-CeOx nanoclusters due to its large specific surface area, outstanding electrical conductivity, light weight, and excellent chemical stability [42-47]. Benefiting from the synergistic effect between Co and CeOx, as well as the strong metal-support interaction between Co-CeOx and NGH, the synthesized Co-CeOx/NGH NPs exhibits outstanding catalytic performance for hydrolysis of AB, with a turnover frequency (TOF) value of 79.5 min-1, which is higher than most of the reported non-noble metal based catalysts, and even comparable to some noble metal based catalysts.
Nitrogen-doped graphene hydrogel (NGH) was synthesized at 180 ℃ according to a hydrothermal method and further used to anchor the Co-CeOx NPs by a simple one-step co-reduction method at room temperature. The Co-CeOx/NGH NPs with different compositions were synthesized by altering the amounts of cerous nitrate which were further detected accurately by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). As shown in Table S1 (Supporting information), the ICP-AES results indicate the actual atomic composition of the Co-CeOx/NGH catalysts, which are agree well with the initial atomic molar ratio. In the beginning, the Co/NGH catalyst was synthesized to study for the catalytic performance toward the hydrolysis of ammonia borane at room temperature. As shown in Fig. 1a, the Co/NGH NPs, which needs more than 10 min to release merely less than 2 equivalent of gas, exhibits very poor catalytic activity and hydrogen selectivity, with a TOF value of 6.4 min-1 (Fig. 1b). Whereas, after introducing the Ce to the catalyst, the catalytic activity and hydrogen selectivity of the as-prepared Co-CeOx/NGH catalysts are significantly enhanced. Specifically, Co-(CeOx)0.48/NGH exhibits almost 100% hydrogen selectivity with enhanced TOF value of 60.6 min-1. Further increase the Ce/Co ratios to 1.37 and 2.28, the resulted Co-(CeOx)1.37/NGH, and Co-(CeOx)2.28/NGH both Exhibit 100% hydrogen selectivity with TOF values decreasing to 41.3 and 20.1 min-1, respectively. Among all the catalysts tested, Co- (CeOx)0.91/NGH exhibits the highest catalytic activity and 100% hydrogen selectivity, with a TOF value of 79.5 min-1, which is almost 13 times higher than that of Co/NGH and higher than most of the reported non-noble metals based catalysts, including some noble metal based catalysts as shown in Table S2 (Supporting information). However, there is almost no activity for CeOx/rGO, suggesting the synergistic effect between Co and Ce in facilitating hydrogen generation from hydrolysis of ammonia borane.

|
Download:
|
| Fig. 1. (a, b) Catalytic performance tests of Co-CeOx/rGO with different molar of Ce content. (catalyst/NH3BH3 = 0.04) (a, b, c, d, e and f, represent the Co-(CeOx)0.91/NGH Co-(CeOx)0.48/NGH, Co-(CeOx)1.37/NGH, Co-(CeOx)2.28/NGH, Co/NGH and CeOx/NGH, respectively) (c) corresponding TOF values and H2 selectivity towards the hydrolysis of ammonia borane at 298 K. | |
To study the effects of different supported materials, Co- (CeOx)0.91 anchored on other supports, including reduced graphene oxide (rGO), XC-72 or without supported material, were also synthesized and used for catalyzing the hydrolysis of AB. As shown in Fig. S1 (Supporting information), the Co-(CeOx)0.91/NGH exhibits the highest catalytic activity and 100% hydrogen selectivity among all the tested samples, which suggested that the nitrogen-doped graphene hydrogel played a critical role in accelerating hydrogen generation from hydrolysis of ammonia borane, probably due to the strong metal-support interaction (vide infra).
To obtain the activation energy (Ea) of hydrolysis of ammonia borane catalyzed by Co-(CeOx)0.91/NGH, the temperatures of catalytic decomposition reactions were varied from 298 K to 313 K (Fig. 2a). As shown in Fig. 2b, the Ea was determined to be 31.82 kJ/mol. Moreover, as shown in Fig. 2d, the TOF values of Co- (CeOx)0.91/NGH at 298, 303, 308 and 313 K are measured to be 79.5, 99.4, 111.6 and 152.6 min-1, respectively. Furthermore, in order to study the durability of the Co-(CeOx)0.91/NGH, it was further tested by adding the same molar content of ammonia borane after the completion of previous reactions at 298 K. As shown in Fig. 2c, even after 5 cycles of the catalytic reaction process, the hydrogen selectivity still remained well, with a slight decrease of catalytic activity.

|
Download:
|
| Fig. 2. (a) Time plots for hydrogen generation toward the hydrolysis of ammonia borane catalyzed by Co-(CeOx)0.91/NGH at temperatures ranging from 298 K to 313 K. (b) Arrhenius plot of lnk versus 1/T during the hydrolysis of NH3BH3 over Co-(CeOx)0.91/NGH (c) durability tests of Co-(CeOx)0.91/NGH toward the hydrolysis of ammonia borane at 298 K. (d) corresponding TOF values and H2 selectivity towards the hydrolysis of ammonia borane at different temperatures (298–313 K). | |
The as-obtained Co-(CeOx)/NGH catalysts with different molar contents of CeOx were characterized by powder X-ray diffraction (XRD). As shown in Fig. S2 (Supporting information), for Co/NGH, the characteristic peaks at 44.3° is observed which belong to the fcc (111) diffraction peaks of Co (PDF#15-0806). After introduction of Ce, broad and weak diffraction peaks at around 28.6° are observed which can be assigned to CeO2 (PDF#43-1002). However, these samples display much weaker signals of Co metal than the sample without Ce, probably due to the introduction of Ce, which could disturb the long-range order of the Co metal resulting in the decrease of crystallinity. It has been reported that the features of short-range order and long-range disorder in the amorphous or low degree crystallinity state is beneficial to the boosting catalytic performance [48, 49].
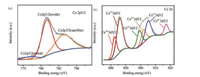
X-ray photoelectron spectroscopy (XPS) was performed to analyze the chemical compositions and surface interaction of Co-(CeOx)0.91/NGH. As shown in Fig. 3a, for Co-(CeOx)0.91/NGH, the Co 2p3/2 peak at the binding energy of 777.43 eV is assigned to the Co0, which has a negative shift of 0.47 eV compared with Co metal [50]. Furthermore, the Co 2p3/2 peaks at the binding energies of 781.59 eV and 786.32 eV are observed which are assigned to the oxidized Co and satellite state, respectively. The formation of oxidized Co most possibly occurred during the catalyst preparation process for the XPS characterization [51]. On the other hand, for the Ce 3d spectrum (Fig. 3b), both signals of Ce3+ and Ce4+ are observed [52]. Specifically, the peaks of Ce 3d3/2 and Ce 3d5/2 at the binding energies of 882.65 and 901.79 eV are assigned to Ce4+, which have positive shifts of 0.35 eV and 0.89 eV compared with pure ceric oxide [53, 54], respectively. Meanwhile, the peaks of Ce 3d3/2 and Ce 3d5/2 at the binding energies of 885.98 eV and 904.54 eV are attributed to Ce3+, which exhibit positive shifts of 0.18 eV and 0.64 eV comparing to pure ceric oxide [55, 56], respectively. These results indicate that there is strong electronic interaction between Co and CeOX, which could further influence the bonding configuration of the metal active center and result in the enhancement of catalytic activity.
|
Download:
|
| Fig. 3. (a) The XPS spectra of (a) Co 2p3/2 and (b) Ce 3d for the Co-(CeOx)0.91/NGH. | |
Furthermore, it is clear that the peaks of intensities of oxygen containing functional groups for the C 1s of Co-(CeOx)0.91/NGH (Fig. S3b in Supporting information), such as —C—O, —C=O, —COO, have weaken in different degrees, comparing to the C 1s of GO (Fig. S3a in Supporting information) [57], suggesting that a large amount of oxygen groups have been reduced. At the same time, a new peak appeared at the binding energies of 285.6 eV, which is attribute to the introduction of nitrogen atoms. On the other hand, as shown in Fig. S4 (Supporting information), for N 1s of Co-(CeOx)0.91/NGH, three types of nitrogen doped species can be observed clearly. More specifically, the peaks at the binding energies of 399.5, 398.2 and 401.1 eV are assigned to pyrrolic N, pyridinic N and graphitic N, respectively [58].
In addition, as shown in Fig. S5a (Supporting information), the N-doped graphene hydrogel is a black cylinder, with the height of 3.6 nm and diameter of 2.2 nm. As shown in Fig. S5b (Supporting information), the NGH displays a three-dimensional poriferous reticulate-like architecture, with varieties of porous sizes. The porous structure could provide abundant of surface active sites for the catalytic hydrogen generation. Furthermore, the porosity of the NGH was detected by N2 adsorption/desorption at 77 K. The specific surface area of the NGH sample is measured to be 190.42 m2/g, with the pore diameter of 3.7 nm, which calculated by the Barrett-Joyner-Halenda method (Fig. S6 in Supporting information). It is reported that these pores on the surface of the NGH could significantly increase the contact between AB molecules and metal active centers, resulting in facilitating release evolved hydrogen bubbles during the catalytic process [59].
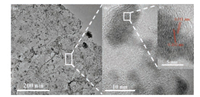
The microstructures of the obtained Co-(CeOx)0.91/NGH NPs were analyzed by transmission electron microscopy (TEM). As shown in Figs. 4a and b, Co-(CeOx)0.91 nanoclusters are well dispersed on the 3D NGH. Furthermore, a lattice fringes with d-spacing of 0.311 nm is observed in the high-resolutionTEM (HRTEM) image (Fig. 4c), which is belong to the (111) plane of CeOx. Similarly, the lattice fringes with d-spacing of 0.202 nm is also observed, corresponding to the (111) plane of Co. In addition, the selected area electron diffraction (SAED) pattern of Co-(CeOx)0.91/NGH further indicates polycrystalline nature of the composite (Fig. S7 in Supporting information). In addition, obvious diffraction rings indexed to (111) plane of Co, and (200) plane of CeO2 are observed. All of the results confirm the coexistence of Co and Ce, which are also consistentwellwith theenergy dispersive X-ray spectrometry result (Fig. S8 in Supporting information). For comparison, Co/NGH was also characterized by TEM (Fig. S9 in Supporting information). However, severely aggregation is observed, which might impede the interaction between the metal active centers and NH3BH3 molecule, resulting in the poor catalytic activity.
|
Download:
|
| Fig. 4. TEM images of different magnifications (a, b) and the magnified HRTEM image in (c) of Co-(CeOx)0.91/NGH. | |
In summary, Co-CeOx nanoclusters anchored on three-dimensional nitrogen doped graphene hydrogel (NGH) was successfully synthesized by a simple one step co-reduction method. Benefiting from strong electron interaction between Co and CeOx, as well as the strongmetal-support interaction between Co-CeOx and 3D NGH, the obtained Co-CeOx/NGH catalyst exhibits superior catalytic performance for hydrolysis of NH3BH3 at room temperature, with a TOF value of 79.5min-1, which is almost 13 times higher than that of Co/ NGH. This simple in situ synthetic method can not only facilitate the promotion of ammonia borane as chemical hydrogen storage material but also develop a new area for the fabrication of other Ce-mixed nanocatalysts for more applications.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (No. 21571145), and Large-scale Instrument and Equipment Sharing Foundation of Wuhan University.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.04.009.
| [1] |
Y. Du, Y.B. Shen, Y.L. Zhan, Chin. Chem. Lett. 28 (2017) 1746-1750. DOI:10.1016/j.cclet.2017.05.018 |
| [2] |
J. Nowotny, T. Bak, D. Chu, et al., Int. J. Hydrogen Energy 39 (2014) 4151-4157. DOI:10.1016/j.ijhydene.2013.12.114 |
| [3] |
M. Pagliaro, A.G. Konstandopoulos, R. Ciriminna, et al., Energy Environ. Sci. 3 (2010) 279-287. DOI:10.1039/b923793n |
| [4] |
A. Boddien, D. Mellmann, F. Gärtner, et al., Science 333 (2011) 1733-1736. DOI:10.1126/science.1206613 |
| [5] |
R. Zeb, L. Salar, U. Awan, et al., Renew. Energy 71 (2014) 123-132. DOI:10.1016/j.renene.2014.05.012 |
| [6] |
J.X. Feng, J.Q. Wu, Y.X. Tong, et al., J. Am. Chem. Soc. 140 (2018) 610-617. DOI:10.1021/jacs.7b08521 |
| [7] |
J.X. Feng, H. Xu, Y.T. Dong, et al., Angew. Chem. Int. Ed. 129 (2017) 3006-3010. DOI:10.1002/ange.201611767 |
| [8] |
J.X. Feng, H. Xu, S.H. Ye, et al., Angew. Chem. Int. Ed. 56 (2017) 8120-8124. DOI:10.1002/anie.v56.28 |
| [9] |
J. Graetz, Chem. Soc. Rev. 38 (2009) 73-82. DOI:10.1039/B718842K |
| [10] |
W. Luo, P.G. Campbell, L.N. Zakharov, et al., J. Am. Chem. Soc. 133 (2011) 19326-19329. DOI:10.1021/ja208834v |
| [11] |
D. Pukazhselvan, V. Kumar, S. Singh, Nano Energy 1 (2012) 566-589. DOI:10.1016/j.nanoen.2012.05.004 |
| [12] |
M.V. Lototskyy, V.A. Yartys, B.G. Pollet, et al., Int. J. Hydrogen Energy 39 (2014) 5818-5851. DOI:10.1016/j.ijhydene.2014.01.158 |
| [13] |
S. Fukuzumi, T. Suenobu, Dalton Trans. 42 (2013) 18-28. DOI:10.1039/C2DT31823G |
| [14] |
Y.L. Zhan, Y.B. Shen, S.P. Li, et al., Chin. Chem. Lett. 28 (2017) 1353-1357. DOI:10.1016/j.cclet.2017.03.038 |
| [15] |
E. Ha, L.Y.S. Lee, J. Wang, et al., Adv. Mater. 26 (2014) 3496-3500. DOI:10.1002/adma.v26.21 |
| [16] |
Q.L. Zhu, Q. Xu, Energy Environ. Sci. 8 (2015) 478-512. DOI:10.1039/C4EE03690E |
| [17] |
M. Yadava, Q. Xu, Energy Environ. Sci. 5 (2012) 9698-9725. DOI:10.1039/c2ee22937d |
| [18] |
Y.N. Men, X.Q. Du, G.Z. Cheng, W. Luo, Int. J. Hydrogen Energy 42 (2017) 27165-27173. DOI:10.1016/j.ijhydene.2017.08.214 |
| [19] |
S.K. Singh, Q. Xu, Chem. Commun. 46 (2010) 6545-6547. DOI:10.1039/c0cc01879a |
| [20] |
S.K. Singh, Q. Xu, Inorg. Chem. 49 (2010) 6148-6152. DOI:10.1021/ic1007654 |
| [21] |
S.K. Singh, Q. Xu, J. Am. Chem. Soc. 131 (2009) 18032-18033. DOI:10.1021/ja908037t |
| [22] |
J.M. Yan, Z.L. Wang, L. Gu, et al., Adv. Energy Mater. 5 (2015) 10-11. |
| [23] |
L. Yang, W. Luo, G.Z. Cheng, Int. J. Hydrogen Energy 41 (2016) 439-446. DOI:10.1016/j.ijhydene.2015.10.074 |
| [24] |
X.Q. Du, C.L. Yang, X. Zeng, et al., Int. J. Hydrogen Energy 42 (2017) 14181-14187. DOI:10.1016/j.ijhydene.2017.04.052 |
| [25] |
L. Yang, N. Cao, C. Du, et al., Mater. Lett. 115 (2014) 113-116. DOI:10.1016/j.matlet.2013.10.039 |
| [26] |
J. Hu, Z. Chen, M. Li, et al., ACS. Appl. Mater. Inter. 6 (2014) 13191-13200. DOI:10.1021/am503037k |
| [27] |
X. Qiu, X. Wu, Y. Wu, Q. Liu, C. Huang, RSC Adv. 6 (2016) 106211-106217. DOI:10.1039/C6RA24000C |
| [28] |
C.Y. Peng, L. Kang, S. Cao, et al., Angew. Chem. Int. Ed. 54 (2015) 15725-15729. DOI:10.1002/anie.201508113 |
| [29] |
C. Tang, F.L. Qu, A.M. Asiri, et al., Inorg. Chem. Front. 4 (2017) 659-662. |
| [30] |
M. Paladini, G.M. Arzac, V. Godinho, M.J. De Haro, A. Fernández, Appl. Catal. B-Environ. 158 (2014) 400-409. |
| [31] |
J.F. Shen, L. Yang, K. Hu, W. Luo, G.Z. Cheng, Int. J. Hydrogen Energy 40 (2015) 1062-1070. DOI:10.1016/j.ijhydene.2014.11.031 |
| [32] |
L. Wen, Z. Zheng, W. Luo, P. Cai, G.Z. Cheng, Chin. Chem. Lett. 26 (2015) 1345-1350. DOI:10.1016/j.cclet.2015.06.019 |
| [33] |
X.Q. Du, S.Y. Tan, P. Cai, W. Luo, G.Z. Cheng, J. Mater. Chem. A 4 (2016) 14572-14576. DOI:10.1039/C6TA05917A |
| [34] |
J.T. Hu, Z.X. Chen, M.X. Li, X.H. Zhou, H.B. Lu, ACS Appl. Mater. Inter. 6 (2014) 13191-13200. DOI:10.1021/am503037k |
| [35] |
L. Yang, N. Cao, C. Du, et al., Mater. Lett. 115 (2014) 113-116. DOI:10.1016/j.matlet.2013.10.039 |
| [36] |
W. Feng, L. Yang, N. Cao, et al., Int. J. Hydrogen Energy 39 (2014) 3371-3380. DOI:10.1016/j.ijhydene.2013.12.113 |
| [37] |
Y.S. Du, N. Cao, L. Yang, W. Luo, G.Z. Cheng, New. J. Chem. 37 (2013) 3035-3042. DOI:10.1039/c3nj00552f |
| [38] |
Y.Q. Ge, P.H. Diao, X. Chen, N.N. Zhang, G. Cheng, Chin. Chem. Lett. (2018), doi: http://dx.doi.org/10.1016/j.cclet.2018.01.002.
|
| [39] |
X.M. Liao, V. Pitchon, P.H. Guong, W. Chu, V. Caps, Chin. Chem. Lett. 28 (2017) 293-296. DOI:10.1016/j.cclet.2016.06.043 |
| [40] |
Y.H. Zhou, S. Wang, Y. Wan, et al., J. Alloy Compd. 728 (2017) 902-909. DOI:10.1016/j.jallcom.2017.09.075 |
| [41] |
Y.Y. Jiang, H.B. Dai, Y.J. Zhong, D.M. Chen, P. Wang, J. Chem. Eur. 21 (2015) 15439-15445. DOI:10.1002/chem.v21.43 |
| [42] |
Y. Zhang, J.Y. Zhu, H.B. Ren, Y.T. Bi, L. Zhang, Chin. Chem. Lett. 28 (2017) 935-942. DOI:10.1016/j.cclet.2017.01.023 |
| [43] |
Y. Xu, Z. Lin, X. Huang, et al., ACS Nano 7 (2013) 4042-4049. DOI:10.1021/nn4000836 |
| [44] |
Y. Li, Y. Zhao, H. Cheng, et al., J. Am. Chem. Soc. 134 (2011) 15-18. |
| [45] |
D.C. Marcano, D.V. Kosynkin, J.M. Berlin, et al., ACS Nano 4 (2010) 4806-4814. DOI:10.1021/nn1006368 |
| [46] |
X.Q. Du, C. Liu, C. Du, et al., Nano Res. 10 (2017) 2856-2865. DOI:10.1007/s12274-017-1494-6 |
| [47] |
Y.N. Men, J. Su, X. Du, et al., J. Alloy Compd. 735 (2018) 1271-1276. DOI:10.1016/j.jallcom.2017.11.137 |
| [48] |
W.D. Chemelewski, H.C. Lee, J.F. Lin, A.J. Bard, C.B. Mullins, J. Am. Chem. Soc. 136 (2014) 2843-2850. DOI:10.1021/ja411835a |
| [49] |
J.M. McEnaney, J.C. Crompton, J.F. Callejas, et al., Chem. Mater. 26 (2014) 4826-4831. DOI:10.1021/cm502035s |
| [50] |
H. Yuan, L. Kong, T. Li, Q. Zhang, Chin. Chem. Lett. 28 (2017) 2180-2194. DOI:10.1016/j.cclet.2017.11.038 |
| [51] |
N. Cao, L. Yang, C. Du, et al., J. Mater. Chem. A 2 (2014) 14344-14347. DOI:10.1039/C4TA02964J |
| [52] |
X. Liu, K. Zhou, L. Wang, B. Wang, Y. Li, J. Am. Chem. Soc. 131 (2009) 3140-3141. DOI:10.1021/ja808433d |
| [53] |
G.M. Ingo, J. Am. Ceram, Soc. 74 (199) (2016) 381-386.
|
| [54] |
A. Garnache, A.A. Kachanov, F. Stoeckel, et al., JOSAB 17 (2000) 1589-1598. DOI:10.1364/JOSAB.17.001589 |
| [55] |
Y. Uwamino, T. Ishizuka, H. Yamatera, J. Electron Spectrosc. 34 (1984) 67-78. DOI:10.1016/0368-2048(84)80060-2 |
| [56] |
G.M. Ingo, J. Am. Ceram. Soc. 74 (1991) 381-386. DOI:10.1111/jace.1991.74.issue-2 |
| [57] |
D.X. Yang, A. Velamakanni, G. Bozoklu, et al., Carbon 47 (2009) 145-152. DOI:10.1016/j.carbon.2008.09.045 |
| [58] |
P. Chen, J.J. Yang, S.S. Li, et al., Nano Energy 2 (2013) 249-256. DOI:10.1016/j.nanoen.2012.09.003 |
| [59] |
X.P. Zhang, D. Liu, L. Yang, L.M. Zhou, T.Y. You, J. Mater. Chem. A 3 (2015) 10031-10037. DOI:10.1039/C5TA00355E |
 2018, Vol. 29
2018, Vol. 29 


